Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease
JAMA ( IF 120.7 ) Pub Date : 2019-11-12 , DOI: 10.1001/jama.2019.16585 Anne C Goldberg 1 , Lawrence A Leiter 2 , Erik S G Stroes 3 , Seth J Baum 4 , Jeffrey C Hanselman 5 , LeAnne T Bloedon 5 , Narendra D Lalwani 5 , Pragna M Patel 5 , Xin Zhao 5, 6 , P Barton Duell 7
JAMA ( IF 120.7 ) Pub Date : 2019-11-12 , DOI: 10.1001/jama.2019.16585 Anne C Goldberg 1 , Lawrence A Leiter 2 , Erik S G Stroes 3 , Seth J Baum 4 , Jeffrey C Hanselman 5 , LeAnne T Bloedon 5 , Narendra D Lalwani 5 , Pragna M Patel 5 , Xin Zhao 5, 6 , P Barton Duell 7
Affiliation
|
Importance
Additional treatment options are needed for patients who do not achieve sufficient reduction in low-density lipoprotein cholesterol (LDL-C) level with available lipid-lowering therapies. Objective
To assess the efficacy of bempedoic acid vs placebo in patients at high cardiovascular risk receiving maximally tolerated lipid-lowering therapy. Design, Setting, and Participants
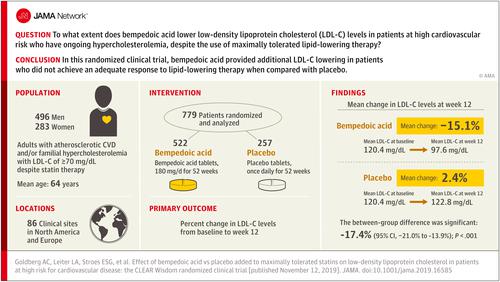
Phase 3, randomized, double-blind, placebo-controlled clinical trial conducted at 91 clinical sites in North America and Europe from November 2016 to September 2018, with a final date of follow-up of September 22, 2018. A total of 779 patients with atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia, or both met randomization criteria, which included LDL-C level 70 mg/dL (1.8 mmol/L) or greater while receiving maximally tolerated lipid-lowering therapy. Interventions
Patients were randomized 2:1 to treatment with bempedoic acid (180 mg) (n = 522) or placebo (n = 257) once daily for 52 weeks. Main Outcomes and Measures
The primary end point was percent change from baseline in LDL-C level at week 12. Secondary measures included changes in levels of lipids, lipoproteins, and biomarkers. Results
Among 779 randomized patients (mean age, 64.3 years; 283 women [36.3%]), 740 (95.0%) completed the trial. At baseline, mean LDL-C level was 120.4 (SD, 37.9) mg/dL. Bempedoic acid lowered LDL-C levels significantly more than placebo at week 12 (-15.1% vs 2.4%, respectively; difference, -17.4% [95% CI, -21.0% to -13.9%]; P < .001). Significant reductions with bempedoic acid vs placebo were observed at week 12 for non-high-density lipoprotein cholesterol (-10.8% vs 2.3%; difference, -13.0% [95% CI, -16.3% to -9.8%]; P < .001), total cholesterol (-9.9% vs 1.3%; difference, -11.2% [95% CI, -13.6% to -8.8%]; P < .001), apolipoprotein B (-9.3% vs 3.7%; difference, -13.0% [95% CI, -16.1% to -9.9%]; P < .001), and high-sensitivity C-reactive protein (median, -18.7% vs -9.4%; difference, -8.7% [asymptotic confidence limits, -17.2% to -0.4%]; P = .04). Common adverse events included nasopharyngitis (5.2% vs 5.1% with bempedoic acid and placebo, respectively), urinary tract infection (5.0% vs 1.9%), and hyperuricemia (4.2% vs 1.9%). Conclusions and Relevance
Among patients at high risk for cardiovascular disease receiving maximally tolerated statins, the addition of bempedoic acid compared with placebo resulted in a significant lowering of LDL-C level over 12 weeks. Further research is needed to assess the durability and clinical effect as well as long-term safety. Trial Registration
ClinicalTrials.gov Identifier: NCT02991118.
中文翻译:

Bempedoic Acid vs 安慰剂添加到最大耐受他汀类药物对心血管疾病高危患者低密度脂蛋白胆固醇的影响
重要性 对于使用可用的降脂疗法未能充分降低低密度脂蛋白胆固醇 (LDL-C) 水平的患者,需要额外的治疗选择。目的评估 bempedoic 酸与安慰剂在接受最大耐受降脂治疗的高心血管风险患者中的疗效。设计、设置和参与者 2016 年 11 月至 2018 年 9 月在北美和欧洲的 91 个临床地点进行的第 3 期随机、双盲、安慰剂对照临床试验,最终随访日期为 2018 年 9 月 22 日. 共有 779 名患有动脉粥样硬化性心血管疾病、杂合子家族性高胆固醇血症或两者都符合随机化标准的患者,其中 LDL-C 水平为 70 mg/dL (1. 8 mmol/L) 或更高,同时接受最大耐受的降脂治疗。干预 患者以 2:1 的比例随机接受苯哌多酸 (180 mg) (n = 522) 或安慰剂 (n = 257) 治疗,每天一次,持续 52 周。主要结果和指标 主要终点是第 12 周时 LDL-C 水平相对于基线的变化百分比。次要指标包括脂质、脂蛋白和生物标志物水平的变化。结果 在 779 名随机患者(平均年龄 64.3 岁;283 名女性 [36.3%])中,740 名(95.0%)完成了试验。在基线时,平均 LDL-C 水平为 120.4 (SD, 37.9) mg/dL。在第 12 周,贝培多酸比安慰剂显着降低了 LDL-C 水平(分别为 -15.1% 和 2.4%;差异,-17.4% [95% CI,-21.0% 到 -13.9%];P < .001)。在第 12 周观察到贝培多酸与安慰剂相比,非高密度脂蛋白胆固醇显着降低(-10.8% 与 2.3%;差异,-13.0% [95% CI,-16.3% 至 -9.8%];P < . 001),总胆固醇(-9.9% 对 1.3%;差异,-11.2% [95% CI,-13.6% 到 -8.8%];P < .001),载脂蛋白 B(-9.3% 对 3.7%;差异, -13.0% [95% CI,-16.1% 至 -9.9%];P < .001)和高敏 C 反应蛋白(中位数,-18.7% 对 -9.4%;差异,-8.7% [渐近置信度)限制,-17.2% 至 -0.4%];P = .04)。常见的不良事件包括鼻咽炎(使用 bempedoic 酸和安慰剂分别为 5.2% 和 5.1%)、尿路感染(5.0% 对 1.9%)和高尿酸血症(4.2% 对 1.9%)。结论和相关性 在接受最大耐受他汀类药物治疗的心血管疾病高危患者中,与安慰剂相比,添加 bempedoic 酸导致 LDL-C 水平在 12 周内显着降低。需要进一步的研究来评估耐久性和临床效果以及长期安全性。试验注册 ClinicalTrials.gov 标识符:NCT02991118。
更新日期:2019-11-12
中文翻译:

Bempedoic Acid vs 安慰剂添加到最大耐受他汀类药物对心血管疾病高危患者低密度脂蛋白胆固醇的影响
重要性 对于使用可用的降脂疗法未能充分降低低密度脂蛋白胆固醇 (LDL-C) 水平的患者,需要额外的治疗选择。目的评估 bempedoic 酸与安慰剂在接受最大耐受降脂治疗的高心血管风险患者中的疗效。设计、设置和参与者 2016 年 11 月至 2018 年 9 月在北美和欧洲的 91 个临床地点进行的第 3 期随机、双盲、安慰剂对照临床试验,最终随访日期为 2018 年 9 月 22 日. 共有 779 名患有动脉粥样硬化性心血管疾病、杂合子家族性高胆固醇血症或两者都符合随机化标准的患者,其中 LDL-C 水平为 70 mg/dL (1. 8 mmol/L) 或更高,同时接受最大耐受的降脂治疗。干预 患者以 2:1 的比例随机接受苯哌多酸 (180 mg) (n = 522) 或安慰剂 (n = 257) 治疗,每天一次,持续 52 周。主要结果和指标 主要终点是第 12 周时 LDL-C 水平相对于基线的变化百分比。次要指标包括脂质、脂蛋白和生物标志物水平的变化。结果 在 779 名随机患者(平均年龄 64.3 岁;283 名女性 [36.3%])中,740 名(95.0%)完成了试验。在基线时,平均 LDL-C 水平为 120.4 (SD, 37.9) mg/dL。在第 12 周,贝培多酸比安慰剂显着降低了 LDL-C 水平(分别为 -15.1% 和 2.4%;差异,-17.4% [95% CI,-21.0% 到 -13.9%];P < .001)。在第 12 周观察到贝培多酸与安慰剂相比,非高密度脂蛋白胆固醇显着降低(-10.8% 与 2.3%;差异,-13.0% [95% CI,-16.3% 至 -9.8%];P < . 001),总胆固醇(-9.9% 对 1.3%;差异,-11.2% [95% CI,-13.6% 到 -8.8%];P < .001),载脂蛋白 B(-9.3% 对 3.7%;差异, -13.0% [95% CI,-16.1% 至 -9.9%];P < .001)和高敏 C 反应蛋白(中位数,-18.7% 对 -9.4%;差异,-8.7% [渐近置信度)限制,-17.2% 至 -0.4%];P = .04)。常见的不良事件包括鼻咽炎(使用 bempedoic 酸和安慰剂分别为 5.2% 和 5.1%)、尿路感染(5.0% 对 1.9%)和高尿酸血症(4.2% 对 1.9%)。结论和相关性 在接受最大耐受他汀类药物治疗的心血管疾病高危患者中,与安慰剂相比,添加 bempedoic 酸导致 LDL-C 水平在 12 周内显着降低。需要进一步的研究来评估耐久性和临床效果以及长期安全性。试验注册 ClinicalTrials.gov 标识符:NCT02991118。



























 京公网安备 11010802027423号
京公网安备 11010802027423号