当前位置:
X-MOL 学术
›
J. Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Are prescribers not aware of cardiovascular contraindications for diclofenac? A claims data analysis.
Journal of Internal Medicine ( IF 11.1 ) Pub Date : 2019-11-11 , DOI: 10.1111/joim.12990 O Scholle 1 , B Kollhorst 2 , U Haug 1, 3
Journal of Internal Medicine ( IF 11.1 ) Pub Date : 2019-11-11 , DOI: 10.1111/joim.12990 O Scholle 1 , B Kollhorst 2 , U Haug 1, 3
Affiliation
|
OBJECTIVES
To compare diclofenac use before and after implementation of European risk minimization measures in 2013, focusing on diclofenac initiators and prevalence of congestive heart failure (NYHA class II-IV), ischaemic heart disease, peripheral arterial disease and cerebrovascular disease (new contraindications) in these patients in Germany.
METHODS
We included adults with health insurance coverage on 1 January 2011 (cohort 2011) or 1 January 2014 (cohort 2014) and during a 1-year pre-observation period. We defined diclofenac initiators as persons filling a prescription of systemic diclofenac in 2011 (cohort 2011) or 2014 (cohort 2014) and without such a prescription during the respective pre-observation period.
RESULTS
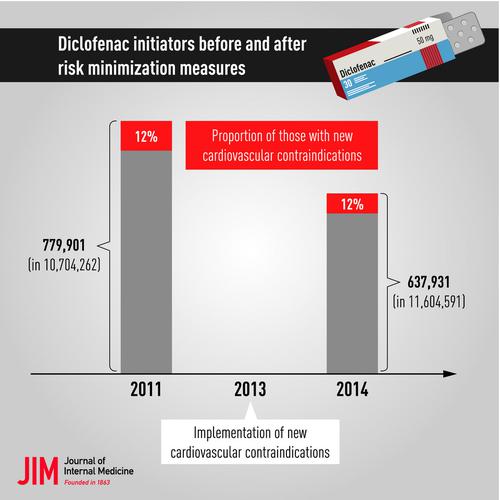
Each cohort comprised >10 million persons. Between 2011 and 2014, the age-standardized proportion of persons initiating diclofenac decreased by 29% (from 8.2% to 5.8%) amongst female patients and by 26% (from 8.5% to 6.3%) amongst male patients; in the subgroup of persons with new contraindications, this proportion decreased by 33% (from 9.8% to 6.6%) amongst female patients and by 31% (from 10.0% to 6.7%) amongst male patients. Amongst diclofenac initiators, the proportion of those with new contraindications did not change between 2011 (12.0%) and 2014 (11.8%).
CONCLUSION
The overall decline of about 30% in diclofenac initiation between 2011 and 2014 was largely independent of the presence or absence of new contraindications. The proportion of diclofenac initiators with a new contraindication remained at a high level (more than one in ten patients), demonstrating the need for research at the prescriber level (e.g. interventional studies) and further measures to improve patient safety.
中文翻译:

处方者是否不了解双氯芬酸的心血管禁忌症?索赔数据分析。
目的比较2013年实施欧洲最小化风险措施前后双氯芬酸的使用情况,重点关注双氯芬酸的引发剂和充血性心力衰竭(NYHA II-IV级),缺血性心脏病,外周动脉疾病和脑血管疾病(新的禁忌症)的患病率这些病人在德国。方法我们纳入了在2011年1月1日(2011年队列)或2014年1月1日(2014年队列)和一年观察期前有健康保险的成年人。我们将双氯芬酸引发剂定义为在2011年(2011年队列)或2014年(2014年队列)服满全身性双氯芬酸处方的人,并且在各自的预观察期中没有此类处方。结果每个队列包括超过1000万人。在2011年至2014年之间,女性患者中双氯芬酸的起始年龄标准化比例下降了29%(从8.2%降至5.8%),男性患者中降低了26%(从8.5%降至6.3%);在具有新禁忌症的亚组中,女性患者的这一比例下降了33%(从9.8%降至6.6%),而男性患者则下降了31%(从10.0%降至6.7%)。在双氯芬酸引发剂中,有新禁忌症的比例在2011年(12.0%)和2014年(11.8%)之间没有变化。结论2011年至2014年,双氯芬酸开始治疗的总体下降约30%,在很大程度上与是否存在新的禁忌症无关。具有新禁忌症的双氯芬酸引发剂的比例仍处于较高水平(十分之一以上的患者),
更新日期:2019-11-13
中文翻译:

处方者是否不了解双氯芬酸的心血管禁忌症?索赔数据分析。
目的比较2013年实施欧洲最小化风险措施前后双氯芬酸的使用情况,重点关注双氯芬酸的引发剂和充血性心力衰竭(NYHA II-IV级),缺血性心脏病,外周动脉疾病和脑血管疾病(新的禁忌症)的患病率这些病人在德国。方法我们纳入了在2011年1月1日(2011年队列)或2014年1月1日(2014年队列)和一年观察期前有健康保险的成年人。我们将双氯芬酸引发剂定义为在2011年(2011年队列)或2014年(2014年队列)服满全身性双氯芬酸处方的人,并且在各自的预观察期中没有此类处方。结果每个队列包括超过1000万人。在2011年至2014年之间,女性患者中双氯芬酸的起始年龄标准化比例下降了29%(从8.2%降至5.8%),男性患者中降低了26%(从8.5%降至6.3%);在具有新禁忌症的亚组中,女性患者的这一比例下降了33%(从9.8%降至6.6%),而男性患者则下降了31%(从10.0%降至6.7%)。在双氯芬酸引发剂中,有新禁忌症的比例在2011年(12.0%)和2014年(11.8%)之间没有变化。结论2011年至2014年,双氯芬酸开始治疗的总体下降约30%,在很大程度上与是否存在新的禁忌症无关。具有新禁忌症的双氯芬酸引发剂的比例仍处于较高水平(十分之一以上的患者),



























 京公网安备 11010802027423号
京公网安备 11010802027423号