Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-11-11 , DOI: 10.1016/s0140-6736(19)32489-4 Féline P B Kroon 1 , Marion C Kortekaas 1 , Annelies Boonen 2 , Stefan Böhringer 3 , Monique Reijnierse 4 , Frits R Rosendaal 5 , Naghmeh Riyazi 6 , Mirian Starmans 7 , Franktien Turkstra 8 , Jende van Zeben 9 , Cornelia F Allaart 1 , Margreet Kloppenburg 10
The Lancet ( IF 168.9 ) Pub Date : 2019-11-11 , DOI: 10.1016/s0140-6736(19)32489-4 Féline P B Kroon 1 , Marion C Kortekaas 1 , Annelies Boonen 2 , Stefan Böhringer 3 , Monique Reijnierse 4 , Frits R Rosendaal 5 , Naghmeh Riyazi 6 , Mirian Starmans 7 , Franktien Turkstra 8 , Jende van Zeben 9 , Cornelia F Allaart 1 , Margreet Kloppenburg 10
Affiliation
|
BACKGROUND
Hand osteoarthritis is a prevalent joint condition that has a high burden of disease and an unmet medical need for effective therapeutic options. Since local inflammation is recognised as contributing to osteoarthritic complaints, the Hand Osteoarthritis Prednisolone Efficacy (HOPE) study aimed to investigate the efficacy and safety of short-term prednisolone in patients with painful hand osteoarthritis and synovial inflammation.
METHODS
The HOPE study is a double-blind, randomised, placebo-controlled trial. We recruited eligible adults from rheumatology outpatient clinics at two sites in the Netherlands. Patients were considered eligible if they had symptomatic hand osteoarthritis and signs of inflammation in their distal and proximal interphalangeal (DIP/PIP) joints. For inclusion, patients were required to have four or more DIP/PIP joints with osteoarthritic nodes; at least one DIP/PIP joint with soft swelling or erythema; at least one DIP/PIP joint with a positive power Doppler signal or synovial thickening of at least grade 2 on ultrasound; and finger pain of at least 30 mm on a 100-mm visual analogue scale (VAS) that flared up during a 48-h non-steroidal anti-inflammatory drug (NSAID) washout (defined as worsening of finger pain by at least 20 mm on the VAS). Eligible patients were randomly assigned (1:1) to receive 10 mg prednisolone or placebo orally once daily for 6 weeks, followed by a 2-week tapering scheme, and a 6-week follow-up without study medication. The patients and study team were masked to treatment assignment. The primary endpoint was finger pain, assessed on a VAS, at 6 weeks in participants who had been randomly assigned to groups and attended the baseline visit. This study is registered with the Netherlands Trial Registry, number NTR5263.
FINDINGS
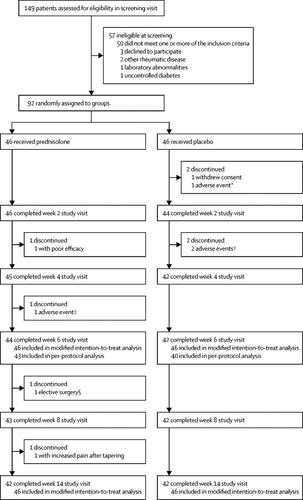
We screened patients for enrolment between Dec 3, 2015, and May 31, 2018. Patients completed baseline visits and started treatment between Dec 14, 2015, and July 2, 2018, and the last study visit of the last patient was Oct 4, 2018. Of 149 patients assessed for eligibility, 57 (38%) patients were excluded (predominantly because they did not meet one or several inclusion criteria, most often because of an absence of synovial inflammation or of flare-ups after NSAID washout) and 92 (62%) patients were eligible for inclusion. We randomly assigned 46 (50%) patients to receive prednisolone and 46 (50%) patients to receive placebo, all of whom were included in the modified intention-to-treat analysis of the primary endpoint. 42 (91%) patients in the prednisolone group and 42 (91%) in the placebo group completed the 14-week study. The mean change between baseline and week 6 on VAS-reported finger pain was -21·5 (SD 21·7) in the prednisolone group and -5·2 (24·3) in the placebo group, with a mean between-group difference (of prednisolone vs placebo) of -16·5 (95% CI -26·1 to -6·9; p=0·0007). The number of non-serious adverse events was similar between the groups. Five serious adverse events were reported during our study: one serious adverse event in the prednisolone group (a myocardial infarction) and four serious adverse events in the placebo group (an infected traumatic leg haematoma that required surgery, bowel surgery, atrial fibrillation that required a pacemaker implantation, and symptomatic uterine myomas that required a hysterectomy). Four (4%) patients discontinued the study because of an adverse event: one (2%) patient receiving prednisolone (for a myocardial infarction) and three (7%) patients receiving placebo (for surgery of the bowel and for an infected leg haematoma and for Lyme disease arthritis of the knee).
INTERPRETATION
Treatment with 10 mg prednisolone for 6 weeks is efficacious and safe for the treatment of patients with painful hand osteoarthritis and signs of inflammation. The results of our study provide clinicians with a new short-term treatment option for patients with hand osteoarthritis who report a flare-up of their disease.
FUNDING
Dutch Arthritis Society.
中文翻译:

手部骨关节炎(HOPE)患者接受10 mg强的松龙治疗6周的结果:一项双盲,随机,安慰剂对照试验。
背景技术手部骨关节炎是一种普遍的关节疾病,其具有高的疾病负担并且对有效治疗选择的医疗需求尚未得到满足。由于公认局部炎症是导致骨关节炎的原因,因此手骨关节炎泼尼松龙功效(HOPE)研究旨在研究短期泼尼松龙在疼痛性手部骨关节炎和滑膜炎患者中的疗效和安全性。方法HOPE研究是一项双盲,随机,安慰剂对照试验。我们从荷兰的两个地点的风湿病门诊招募了合格的成年人。如果患者有症状性手部骨关节炎以及远端和近端指间关节(DIP / PIP)关节发炎的迹象,则认为该患者合格。为了包容,患者需要有四个或更多带有骨关节炎结点的DIP / PIP关节;至少一个DIP / PIP关节有软肿或红斑; 至少一个DIP / PIP关节在超声上具有正功率多普勒信号或滑膜增厚至少2级; 100毫米视觉模拟量表(VAS)上至少30毫米的手指疼痛,并在48小时非甾体类抗炎药(NSAID)冲洗期间发作(定义为手指疼痛加剧至少20毫米)在VAS上)。符合条件的患者被随机分配(1:1),每天口服一次10 mg泼尼松龙或安慰剂,持续6周,然后进行2周的减量方案和6周的随访,而无需研究药物。患者和研究团队被掩盖了治疗任务。主要终点是手指疼痛,通过VAS进行评估,在第6周的参与者中,他们被随机分配到各组并参加了基线访视。该研究已在荷兰审判注册处注册,编号为NTR5263。结果我们筛选了2015年12月3日至2018年5月31日之间的患者入组。患者完成了基线访视并在2015年12月14日至2018年7月2日之间开始治疗,最后一位患者的最后一次研究访视是10月4日。 2018年。在评估合格的149例患者中,有57例(38%)被排除在外(主要是因为他们不符合一项或多项入选标准,最常见的原因是没有滑膜炎症或NSAID冲洗后出现了突然发作)和92例(62%)患者符合纳入条件。我们随机分配了46(50%)名患者接受泼尼松龙和46(50%)名患者接受安慰剂,所有这些都包括在主要终点的修改后的意向性治疗分析中。泼尼松龙组中的42名患者(91%),安慰剂组中的42名患者(91%)完成了为期14周的研究。泼尼松龙组的VAS报告的手指疼痛在基线与第6周之间的平均变化为-21·5(SD 21·7),安慰剂组为-5·2(24·3),组间平均值(泼尼松龙与安慰剂的差)为-16·5(95%CI -26·1至-6·9; p = 0·0007)。两组之间的非严重不良事件数量相似。我们的研究报告了五种严重不良事件:泼尼松龙组(心肌梗死)中的一项严重不良事件和安慰剂组(需要手术,肠道手术,需要起搏器植入的房颤和需要子宫切除术的有症状子宫肌瘤)。四(4%)位患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。四(4%)名患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。四(4%)位患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。一名(2%)患者接受泼尼松龙治疗(用于心肌梗塞),三名(7%)患者接受安慰剂(用于肠外科手术,腿部血肿感染和膝盖莱姆病关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。一名(2%)患者接受泼尼松龙治疗(用于心肌梗塞),三名(7%)患者接受安慰剂(用于肠外科手术,腿部血肿感染和膝盖莱姆病关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。
更新日期:2019-11-29
中文翻译:

手部骨关节炎(HOPE)患者接受10 mg强的松龙治疗6周的结果:一项双盲,随机,安慰剂对照试验。
背景技术手部骨关节炎是一种普遍的关节疾病,其具有高的疾病负担并且对有效治疗选择的医疗需求尚未得到满足。由于公认局部炎症是导致骨关节炎的原因,因此手骨关节炎泼尼松龙功效(HOPE)研究旨在研究短期泼尼松龙在疼痛性手部骨关节炎和滑膜炎患者中的疗效和安全性。方法HOPE研究是一项双盲,随机,安慰剂对照试验。我们从荷兰的两个地点的风湿病门诊招募了合格的成年人。如果患者有症状性手部骨关节炎以及远端和近端指间关节(DIP / PIP)关节发炎的迹象,则认为该患者合格。为了包容,患者需要有四个或更多带有骨关节炎结点的DIP / PIP关节;至少一个DIP / PIP关节有软肿或红斑; 至少一个DIP / PIP关节在超声上具有正功率多普勒信号或滑膜增厚至少2级; 100毫米视觉模拟量表(VAS)上至少30毫米的手指疼痛,并在48小时非甾体类抗炎药(NSAID)冲洗期间发作(定义为手指疼痛加剧至少20毫米)在VAS上)。符合条件的患者被随机分配(1:1),每天口服一次10 mg泼尼松龙或安慰剂,持续6周,然后进行2周的减量方案和6周的随访,而无需研究药物。患者和研究团队被掩盖了治疗任务。主要终点是手指疼痛,通过VAS进行评估,在第6周的参与者中,他们被随机分配到各组并参加了基线访视。该研究已在荷兰审判注册处注册,编号为NTR5263。结果我们筛选了2015年12月3日至2018年5月31日之间的患者入组。患者完成了基线访视并在2015年12月14日至2018年7月2日之间开始治疗,最后一位患者的最后一次研究访视是10月4日。 2018年。在评估合格的149例患者中,有57例(38%)被排除在外(主要是因为他们不符合一项或多项入选标准,最常见的原因是没有滑膜炎症或NSAID冲洗后出现了突然发作)和92例(62%)患者符合纳入条件。我们随机分配了46(50%)名患者接受泼尼松龙和46(50%)名患者接受安慰剂,所有这些都包括在主要终点的修改后的意向性治疗分析中。泼尼松龙组中的42名患者(91%),安慰剂组中的42名患者(91%)完成了为期14周的研究。泼尼松龙组的VAS报告的手指疼痛在基线与第6周之间的平均变化为-21·5(SD 21·7),安慰剂组为-5·2(24·3),组间平均值(泼尼松龙与安慰剂的差)为-16·5(95%CI -26·1至-6·9; p = 0·0007)。两组之间的非严重不良事件数量相似。我们的研究报告了五种严重不良事件:泼尼松龙组(心肌梗死)中的一项严重不良事件和安慰剂组(需要手术,肠道手术,需要起搏器植入的房颤和需要子宫切除术的有症状子宫肌瘤)。四(4%)位患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。四(4%)名患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。四(4%)位患者因不良事件而中止了研究:一名(2%)患者接受泼尼松龙(用于心肌梗死),三名(7%)患者接受安慰剂(用于肠外科手术和腿部血肿感染)以及莱姆病膝盖关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。一名(2%)患者接受泼尼松龙治疗(用于心肌梗塞),三名(7%)患者接受安慰剂(用于肠外科手术,腿部血肿感染和膝盖莱姆病关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。一名(2%)患者接受泼尼松龙治疗(用于心肌梗塞),三名(7%)患者接受安慰剂(用于肠外科手术,腿部血肿感染和膝盖莱姆病关节炎)。解释用10毫克泼尼松龙治疗6周对于痛苦的手部骨关节炎和炎症迹象的患者是有效和安全的。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。我们的研究结果为报告疾病发作的手部骨关节炎患者提供了新的短期治疗方案。资助荷兰关节炎学会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号