Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Testosterone replacement in young male cancer survivors: A 6-month double-blind randomised placebo-controlled trial.
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-11-12 , DOI: 10.1371/journal.pmed.1002960 Jennifer S Walsh 1 , Helen Marshall 2 , Isabelle L Smith 2 , Diana M Greenfield 3 , Jayne Swain 2 , Emma Best 2 , James Ashton 4 , Julia M Brown 2 , Robert Huddart 5 , Robert E Coleman 1 , John A Snowden 6 , Richard J Ross 1
PLOS Medicine ( IF 15.8 ) Pub Date : 2019-11-12 , DOI: 10.1371/journal.pmed.1002960 Jennifer S Walsh 1 , Helen Marshall 2 , Isabelle L Smith 2 , Diana M Greenfield 3 , Jayne Swain 2 , Emma Best 2 , James Ashton 4 , Julia M Brown 2 , Robert Huddart 5 , Robert E Coleman 1 , John A Snowden 6 , Richard J Ross 1
Affiliation
|
BACKGROUND
Young male cancer survivors have lower testosterone levels, higher fat mass, and worse quality of life (QoL) than age-matched healthy controls. Low testosterone in cancer survivors can be due to orchidectomy or effects of chemotherapy and radiotherapy. We have undertaken a double-blind, placebo-controlled, 6-month trial of testosterone replacement in young male cancer survivors with borderline low testosterone (7-12 nmol/l).
METHODS AND FINDINGS
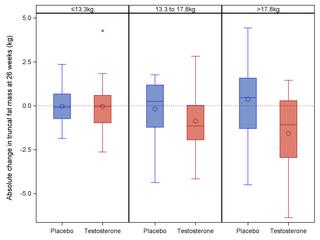
This was a multicentre United Kingdom study conducted in secondary care hospital outpatients. Male survivors of testicular cancer, lymphoma, and leukaemia aged 25-50 years with morning total serum testosterone 7-12 nmol/l were recruited. A total of 136 men were randomised between July 2012 and February 2015 (42.6% aged 25-37 years, 57.4% 38-50 years, 88% testicular cancer, 10% lymphoma, matched for body mass index [BMI]). Participants were randomised 1:1 to receive testosterone (Tostran 2% gel) or placebo for 26 weeks. A dose titration was performed after 2 weeks. The coprimary end points were trunk fat mass and SF36 Physical Functioning score (SF36-PF) at 26 weeks by intention to treat. At 26 weeks, testosterone treatment compared with placebo was associated with decreased trunk fat mass (-0.9 kg, 95% CI -1.6 to -0.3, p = 0.0073), decreased whole-body fat mass (-1.8 kg, 95% CI -2.9 to -0.7, p = 0.0016), and increased lean body mass (1.5 kg, 95% CI 0.9-2.1, p < 0.001). Decrease in fat mass was greatest in those with a high truncal fat mass at baseline. There was no treatment effect on SF36-PF or any other QoL scores. Testosterone treatment was well tolerated. The limitations of our study were as follows: a relatively short duration of treatment, only three cancer groups included, and no hard end point data such as cardiovascular events.
CONCLUSIONS
In young male cancer survivors with low-normal morning total serum testosterone, replacement with testosterone is associated with an improvement in body composition.
TRIAL REGISTRATION
ISRCTN: 70274195, EudraCT: 2011-000677-31.
中文翻译:

年轻男性癌症幸存者中的睾丸激素替代:一项为期6个月的双盲随机安慰剂对照试验。
背景技术与年龄匹配的健康对照相比,年轻的男性男性癌症幸存者睾丸激素水平较低,脂肪量较高且生活质量(QoL)较差。癌症幸存者的睾丸激素水平低可能是由于兰花切除术或化学疗法和放射疗法的影响。我们进行了一项双盲,安慰剂对照,为期6个月的睾丸激素替代治疗,研究对象为边缘性低睾丸激素(7-12 nmol / l)的年轻男性癌症幸存者。方法和研究结果这是在英国二级保健医院门诊进行的一项多中心研究。招募年龄在25至50岁之间的睾丸癌,淋巴瘤和白血病的男性幸存者,早上血清总睾丸激素水平为7-12 nmol / l。在2012年7月至2015年2月之间,共有136名男性被随机分组(42.6%的25-37岁年龄段,57.4%的38-50岁年龄段,88%的睾丸癌,10%的淋巴瘤,符合体重指数[BMI])。参与者以1:1的比例随机接受26周的睾丸激素(Tostran 2%凝胶)或安慰剂。2周后进行剂量滴定。主要终点为26周时的主干脂肪量和SF36身体功能评分(SF36-PF)。在26周时,与安慰剂相比,睾丸激素治疗与躯干脂肪量减少(-0.9 kg,95%CI -1.6至-0.3,p = 0.0073),全身脂肪量减少(-1.8 kg,95%CI- 2.9至-0.7,p = 0.0016)和增加的瘦体重(1.5 kg,95%CI 0.9-2.1,p <0.001)。在基线时具有较高的截断型脂肪量的人中,脂肪量的减少最大。对SF36-PF或任何其他QoL评分均无治疗效果。睾丸激素治疗耐受性良好。我们研究的局限性如下:相对较短的治疗时间,仅包括三个癌症组,并且没有硬终点数据,例如心血管事件。结论在早晨血清总睾丸激素水平低于正常的年轻男性癌症幸存者中,用睾丸激素替代可改善人体成分。试用注册证号:70274195,EudraCT:2011-000677-31。
更新日期:2019-12-03
中文翻译:

年轻男性癌症幸存者中的睾丸激素替代:一项为期6个月的双盲随机安慰剂对照试验。
背景技术与年龄匹配的健康对照相比,年轻的男性男性癌症幸存者睾丸激素水平较低,脂肪量较高且生活质量(QoL)较差。癌症幸存者的睾丸激素水平低可能是由于兰花切除术或化学疗法和放射疗法的影响。我们进行了一项双盲,安慰剂对照,为期6个月的睾丸激素替代治疗,研究对象为边缘性低睾丸激素(7-12 nmol / l)的年轻男性癌症幸存者。方法和研究结果这是在英国二级保健医院门诊进行的一项多中心研究。招募年龄在25至50岁之间的睾丸癌,淋巴瘤和白血病的男性幸存者,早上血清总睾丸激素水平为7-12 nmol / l。在2012年7月至2015年2月之间,共有136名男性被随机分组(42.6%的25-37岁年龄段,57.4%的38-50岁年龄段,88%的睾丸癌,10%的淋巴瘤,符合体重指数[BMI])。参与者以1:1的比例随机接受26周的睾丸激素(Tostran 2%凝胶)或安慰剂。2周后进行剂量滴定。主要终点为26周时的主干脂肪量和SF36身体功能评分(SF36-PF)。在26周时,与安慰剂相比,睾丸激素治疗与躯干脂肪量减少(-0.9 kg,95%CI -1.6至-0.3,p = 0.0073),全身脂肪量减少(-1.8 kg,95%CI- 2.9至-0.7,p = 0.0016)和增加的瘦体重(1.5 kg,95%CI 0.9-2.1,p <0.001)。在基线时具有较高的截断型脂肪量的人中,脂肪量的减少最大。对SF36-PF或任何其他QoL评分均无治疗效果。睾丸激素治疗耐受性良好。我们研究的局限性如下:相对较短的治疗时间,仅包括三个癌症组,并且没有硬终点数据,例如心血管事件。结论在早晨血清总睾丸激素水平低于正常的年轻男性癌症幸存者中,用睾丸激素替代可改善人体成分。试用注册证号:70274195,EudraCT:2011-000677-31。



























 京公网安备 11010802027423号
京公网安备 11010802027423号