当前位置:
X-MOL 学术
›
Exp. Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neurotrophic and neuroprotective effects of a monomeric GLP-1/GIP/Gcg receptor triagonist in cellular and rodent models of mild traumatic brain injury.
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.expneurol.2019.113113 Yazhou Li 1 , Elliot J Glotfelty 2 , Inbar Namdar 3 , David Tweedie 1 , Lars Olson 4 , Barry J Hoffer 5 , Richard D DiMarchi 6 , Chagi G Pick 3 , Nigel H Greig 1
Experimental Neurology ( IF 5.3 ) Pub Date : 2019-11-12 , DOI: 10.1016/j.expneurol.2019.113113 Yazhou Li 1 , Elliot J Glotfelty 2 , Inbar Namdar 3 , David Tweedie 1 , Lars Olson 4 , Barry J Hoffer 5 , Richard D DiMarchi 6 , Chagi G Pick 3 , Nigel H Greig 1
Affiliation
|
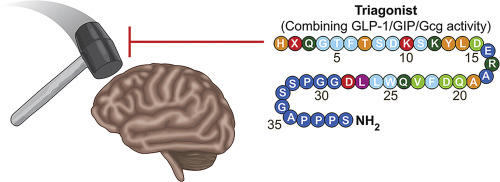
A synthetic monomeric peptide triple receptor agonist, termed "Triagonist" that incorporates glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and glucagon (Gcg) actions, was previously developed to improve upon metabolic and glucose regulatory benefits of single and dual receptor agonists in rodent models of diet-induced obesity and type 2 diabetes. In the current study, the neurotrophic and neuroprotective actions of this Triagonist were probed in cellular and mouse models of mild traumatic brain injury (mTBI), a prevalent cause of neurodegeneration in both the young and elderly. Triagonist dose- and time-dependently elevated cyclic AMP levels in cultured human SH-SY5Y neuronal cells, and induced neurotrophic and neuroprotective actions, mitigating oxidative stress and glutamate excitotoxicity. These actions were inhibited only by the co-administration of antagonists for all three receptor types, indicating the balanced co-involvement of GLP-1, GIP and Gcg receptors. To evaluate physiological relevance, a clinically translatable dose of Triagonist was administered subcutaneously, once daily for 7 days, to mice following a 30 g weight drop close head injury. Triagonist fully mitigated mTBI-induced visual and spatial memory deficits, evaluated at 7 and 30 days post injury. These results establish Triagonist as a novel neurotrophic/protective agent worthy of further evaluation as a TBI treatment strategy.
中文翻译:

单体 GLP-1/GIP/Gcg 受体三激动剂在轻度创伤性脑损伤的细胞和啮齿动物模型中的神经营养和神经保护作用。
一种合成的单体肽三重受体激动剂,称为“Triagonist”,它结合了胰高血糖素样肽-1 (GLP-1)、葡萄糖依赖性促胰岛素多肽 (GIP) 和胰高血糖素 (Gcg) 作用,以前被开发用于改善代谢和葡萄糖单受体激动剂和双受体激动剂在饮食诱导的肥胖和 2 型糖尿病啮齿动物模型中的调节作用。在当前的研究中,这种 Triagonist 的神经营养和神经保护作用在轻度创伤性脑损伤 (mTBI) 的细胞和小鼠模型中进行了探索,mTBI 是年轻人和老年人神经变性的普遍原因。Triagonist 剂量和时间依赖性地升高培养的人类 SH-SY5Y 神经元细胞中的环 AMP 水平,并诱导神经营养和神经保护作用,减轻氧化应激和谷氨酸兴奋性毒性。这些作用仅被所有三种受体类型的拮抗剂共同给药所抑制,表明 GLP-1、GIP 和 Gcg 受体的平衡共同参与。为了评估生理相关性,在 30 g 体重下降闭合性头部损伤后,将临床可转化剂量的 Triagonist 皮下注射给药,每天一次,持续 7 天。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。将临床可转化剂量的 Triagonist 皮下注射给药,每天一次,持续 7 天,小鼠体重下降 30 克,闭合性头部受伤。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。将临床可转化剂量的 Triagonist 皮下给药,每天一次,持续 7 天,小鼠体重下降近 30 克。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。
更新日期:2019-11-13
中文翻译:

单体 GLP-1/GIP/Gcg 受体三激动剂在轻度创伤性脑损伤的细胞和啮齿动物模型中的神经营养和神经保护作用。
一种合成的单体肽三重受体激动剂,称为“Triagonist”,它结合了胰高血糖素样肽-1 (GLP-1)、葡萄糖依赖性促胰岛素多肽 (GIP) 和胰高血糖素 (Gcg) 作用,以前被开发用于改善代谢和葡萄糖单受体激动剂和双受体激动剂在饮食诱导的肥胖和 2 型糖尿病啮齿动物模型中的调节作用。在当前的研究中,这种 Triagonist 的神经营养和神经保护作用在轻度创伤性脑损伤 (mTBI) 的细胞和小鼠模型中进行了探索,mTBI 是年轻人和老年人神经变性的普遍原因。Triagonist 剂量和时间依赖性地升高培养的人类 SH-SY5Y 神经元细胞中的环 AMP 水平,并诱导神经营养和神经保护作用,减轻氧化应激和谷氨酸兴奋性毒性。这些作用仅被所有三种受体类型的拮抗剂共同给药所抑制,表明 GLP-1、GIP 和 Gcg 受体的平衡共同参与。为了评估生理相关性,在 30 g 体重下降闭合性头部损伤后,将临床可转化剂量的 Triagonist 皮下注射给药,每天一次,持续 7 天。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。将临床可转化剂量的 Triagonist 皮下注射给药,每天一次,持续 7 天,小鼠体重下降 30 克,闭合性头部受伤。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。将临床可转化剂量的 Triagonist 皮下给药,每天一次,持续 7 天,小鼠体重下降近 30 克。Triagonist 完全减轻了 mTBI 引起的视觉和空间记忆缺陷,在受伤后 7 天和 30 天进行评估。这些结果将 Triagonist 确立为一种新型神经营养/保护剂,值得进一步评估作为 TBI 治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号