当前位置:
X-MOL 学术
›
Spinal Cord
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study protocol of a double-blind randomised placebo-controlled trial on the effect of a multispecies probiotic on the incidence of antibiotic-associated diarrhoea in persons with spinal cord injury.
Spinal Cord ( IF 2.2 ) Pub Date : 2019-11-11 , DOI: 10.1038/s41393-019-0369-y W X M Faber 1 , J Nachtegaal 2 , J M Stolwijk-Swuste 3 , W J Achterberg-Warmer 4 , C J M Koning 5 , I Besseling-van der Vaart 5 , C A M van Bennekom 1, 2
Spinal Cord ( IF 2.2 ) Pub Date : 2019-11-11 , DOI: 10.1038/s41393-019-0369-y W X M Faber 1 , J Nachtegaal 2 , J M Stolwijk-Swuste 3 , W J Achterberg-Warmer 4 , C J M Koning 5 , I Besseling-van der Vaart 5 , C A M van Bennekom 1, 2
Affiliation
|
STUDY DESIGN
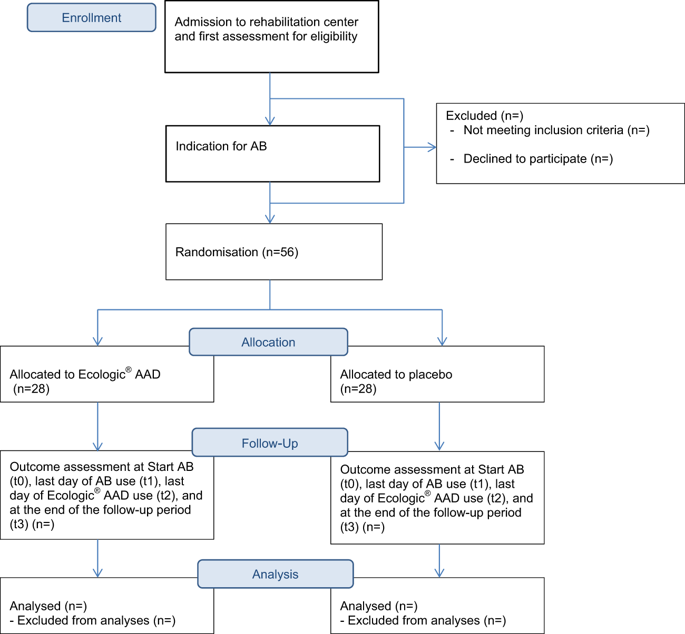
Multi-centre, double-blind randomised placebo-controlled study.
OBJECTIVE
To investigate whether the use of a multispecies probiotic can prevent antibiotic-associated diarrhoea in people with spinal cord injury (SCI).
SETTING
Three Dutch SCI rehabilitation centres.
METHODS
Fifty-six people aged 18-75 years with SCI during inpatient rehabilitation, who require antibiotics, will be given probiotics or placebo randomly assigned (T0). After cessation of the antibiotics (T1), the participants will use probiotics/placebo for 3 more weeks (T2). Defaecation, assessed by the Bristol Stool Scale, and bowel management will be monitored daily until 2 weeks after cessation of probiotics/placebo intake (T3). Also, the degree of nausea and information on quality of life will be collected at T0, T1, T2 and T3.
MAIN OUTCOME MEASURES
The difference between the incidence of antibiotic-associated diarrhoea between people with SCI using probiotics compared to those using a placebo at the moment the antibiotics stops, the probiotics stops and two weeks thereafter.
SECONDARY OUTCOME MEASURES
The time to reach effective bowel management, degree of nausea and quality of life.
REGISTRATION
The Dutch Trial Register- NTR 5831.
中文翻译:

一项关于多物种益生菌对脊髓损伤患者抗生素相关性腹泻发生率影响的双盲随机安慰剂对照试验的研究方案。
研究设计 多中心、双盲随机安慰剂对照研究。目的 调查使用多菌种益生菌是否可以预防脊髓损伤 (SCI) 患者的抗生素相关性腹泻。设置三个荷兰 SCI 康复中心。方法 56 名年龄在 18-75 岁的 SCI 住院康复期间需要抗生素的患者,将随机分配给予益生菌或安慰剂(T0)。停用抗生素后 (T1),参与者将再使用益生菌/安慰剂 3 周 (T2)。通过 Bristol 粪便量表评估的排便和肠道管理将每天监测,直到停止益生菌/安慰剂摄入后 2 周 (T3)。此外,将在 T0、T1、T2 和 T3 收集恶心程度和生活质量信息。主要结果测量 使用益生菌的 SCI 患者与使用安慰剂的患者在抗生素停用、益生菌停用和之后两周之间抗生素相关性腹泻发生率的差异。次要结局指标 达到有效肠道管理的时间、恶心程度和生活质量。注册荷兰审判注册-NTR 5831。
更新日期:2019-11-13
中文翻译:

一项关于多物种益生菌对脊髓损伤患者抗生素相关性腹泻发生率影响的双盲随机安慰剂对照试验的研究方案。
研究设计 多中心、双盲随机安慰剂对照研究。目的 调查使用多菌种益生菌是否可以预防脊髓损伤 (SCI) 患者的抗生素相关性腹泻。设置三个荷兰 SCI 康复中心。方法 56 名年龄在 18-75 岁的 SCI 住院康复期间需要抗生素的患者,将随机分配给予益生菌或安慰剂(T0)。停用抗生素后 (T1),参与者将再使用益生菌/安慰剂 3 周 (T2)。通过 Bristol 粪便量表评估的排便和肠道管理将每天监测,直到停止益生菌/安慰剂摄入后 2 周 (T3)。此外,将在 T0、T1、T2 和 T3 收集恶心程度和生活质量信息。主要结果测量 使用益生菌的 SCI 患者与使用安慰剂的患者在抗生素停用、益生菌停用和之后两周之间抗生素相关性腹泻发生率的差异。次要结局指标 达到有效肠道管理的时间、恶心程度和生活质量。注册荷兰审判注册-NTR 5831。

























 京公网安备 11010802027423号
京公网安备 11010802027423号