Cell Death Discovery ( IF 7 ) Pub Date : 2019-11-12 , DOI: 10.1038/s41420-019-0224-0 Adrian Israel Lehvy , Guy Horev , Yarden Golan , Fabian Glaser , Yael Shammai , Yehuda Gérard Assaraf
|
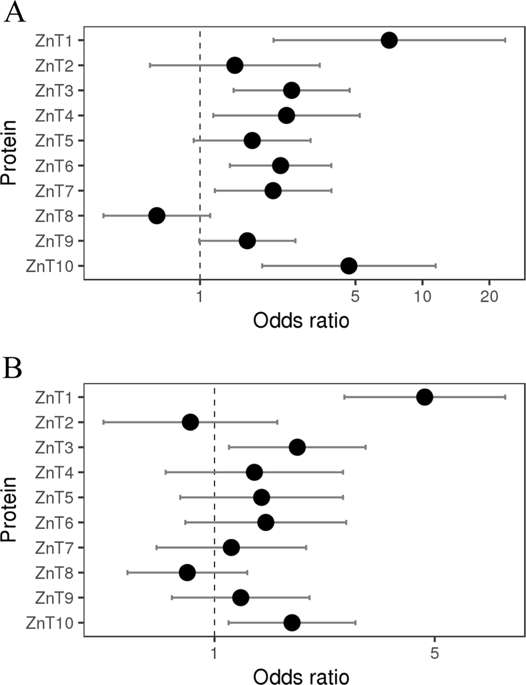
Zinc is vital for the structure and function of ~3000 human proteins and hence plays key physiological roles. Consequently, impaired zinc homeostasis is associated with various human diseases including cancer. Intracellular zinc levels are tightly regulated by two families of zinc transporters: ZIPs and ZnTs; ZIPs import zinc into the cytosol from the extracellular milieu, or from the lumen of organelles into the cytoplasm. In contrast, the vast majority of ZnTs compartmentalize zinc within organelles, whereas the ubiquitously expressed ZnT1 is the sole zinc exporter. Herein, we explored the hypothesis that qualitative and quantitative alterations in ZnT1 activity impair cellular zinc homeostasis in cancer. Towards this end, we first used bioinformatics to analyze inactivating mutations in ZIPs and ZNTs, catalogued in the COSMIC and gnomAD databases, representing tumor specimens and healthy population controls, respectively. ZnT1, ZnT10, ZIP8, and ZIP10 showed extremely high rates of loss of function mutations in cancer as compared to healthy controls. Analysis of the putative functional impact of missense mutations in ZnT1-ZnT10 and ZIP1-ZIP14, using homologous protein alignment and structural predictions, revealed that ZnT1 displays a markedly increased frequency of predicted functionally deleterious mutations in malignant tumors, as compared to a healthy population. Furthermore, examination of ZnT1 expression in 30 cancer types in the TCGA database revealed five tumor types with significant ZnT1 overexpression, which predicted dismal prognosis for cancer patient survival. Novel functional zinc transport assays, which allowed for the indirect measurement of cytosolic zinc levels, established that wild type ZnT1 overexpression results in low intracellular zinc levels. In contrast, overexpression of predicted deleterious ZnT1 missense mutations did not reduce intracellular zinc levels, validating eight missense mutations as loss of function (LoF) mutations. Thus, alterations in ZnT1 expression and LoF mutations in ZnT1 provide a molecular mechanism for impaired zinc homeostasis in cancer formation and/or progression.
中文翻译:

ZnT1表达和功能的改变导致癌症中细胞内锌稳态的受损
锌对于约3000种人类蛋白质的结构和功能至关重要,因此起着关键的生理作用。因此,受损的锌稳态与包括癌症在内的各种人类疾病有关。细胞内锌水平受两个锌转运蛋白家族的严格调控:ZIP和ZnT;锌和锌离子。ZIP将锌从细胞外环境或细胞器腔中导入锌到细胞质中。相比之下,绝大多数ZnT会分隔细胞器中的锌,而无处不在表达的ZnT1是唯一的锌出口国。在本文中,我们探讨了ZnT1活性的定性和定量改变会损害细胞中锌稳态的假说。为此,我们首先使用生物信息学来分析ZIP和ZNT中的失活突变,在COSMIC和gnomAD数据库中分类,分别代表肿瘤标本和健康人群对照。与健康对照组相比,ZnT1,ZnT10,ZIP8和ZIP10在癌症中显示出极高的功能突变丧失率。使用同源蛋白质比对和结构预测,分析ZnT1-ZnT10和ZIP1-ZIP14中的错义突变的假定功能影响,发现与健康人群相比,ZnT1在恶性肿瘤中显示出预测的有害功能突变显着增加的频率。此外,在TCGA数据库中检查了30种癌症类型中ZnT1表达的情况,发现五种具有显着ZnT1过表达的肿瘤类型,预示着癌症患者生存的不良预后。新型功能性锌转运测定 它可以间接测量胞质锌水平,从而确定野生型ZnT1过表达导致细胞内锌水平低。相比之下,预测的有害ZnT1错义突变的过表达并不能降低细胞内锌的水平,从而将八个错义突变确认为功能丧失(LoF)突变。因此,ZnT1表达的改变和ZnT1中的LoF突变为癌症形成和/或发展过程中锌稳态的受损提供了分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号