当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoelectrocatalytic oxidation of 3-pyridinemethanol to 3-pyridinemethanal and vitamin B3 by TiO2 nanotubes
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-11 , DOI: 10.1039/c9cy01583c Sedat Yurdakal 1, 2, 3, 4, 5 , Sıdıka Çetinkaya 1, 2, 3, 4, 5 , Muhsine Beyza Şarlak 1, 2, 3, 4, 5 , Levent Özcan 3, 4, 5, 6, 7 , Vittorio Loddo 8, 9, 10, 11, 12 , Leonardo Palmisano 8, 9, 10, 11, 12
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019-11-11 , DOI: 10.1039/c9cy01583c Sedat Yurdakal 1, 2, 3, 4, 5 , Sıdıka Çetinkaya 1, 2, 3, 4, 5 , Muhsine Beyza Şarlak 1, 2, 3, 4, 5 , Levent Özcan 3, 4, 5, 6, 7 , Vittorio Loddo 8, 9, 10, 11, 12 , Leonardo Palmisano 8, 9, 10, 11, 12
Affiliation
|
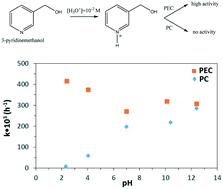
In this paper, the first photoelectrocatalytic (PEC) 3-pyridinemethanol oxidation to 3-pyridinemethanal and vitamin B3 was investigated. To meet this aim, efficient nanotube structured TiO2 on a Ti plate as a photoanode was prepared by an anodic oxidation method in ethylene glycol and characterized by XRD, SEM, and photocurrent techniques. The effect of nanotube morphology, applied potential, Na2SO4 concentration, stirring speed of solution, and pH on the reaction activity and product selectivities were investigated. The TiO2 phase of all of the anodes was mainly the anatase one. The PEC activity, the intensity of the XRD peak and the photocurrent increased by increasing the nanotube length. The activity decreased by decreasing both the Na2SO4 concentration and the applied potential, whereas 3-pyridinemethanal selectivity increased. By increasing the stirring speed of the solution, both the activity and the 3-pyridinemethanal selectivity increased. A lower or no activity was observed for photocatalytic (PC) and electrocatalytic runs, respectively, which were carried out for the sake of comparison. No PC activity was obtained in the presence of N2, but PEC reactions in the presence of N2 were faster than those in the presence of O2. The produced 3-pyridinemethanal in both N2 and O2 atmosphere was reduced at the cathode in the PEC reaction, but its oxidation appeared to be much more favourable. The PC reactions could not be carried out under acidic conditions, whilst the PEC ones could be performed in the pH range of 2–12. Moreover, the results indicate that the PEC method allows higher conversions and selectivities to vitamin B3 to be obtained at pH 7 with respect to those reported in the literature.
中文翻译:

TiO2纳米管将3-吡啶甲醇光催化氧化为3-吡啶甲醛和维生素B3
本文研究了第一个光电催化(PEC)3-吡啶甲醇氧化为3-吡啶甲烷甲醛和维生素B 3的方法。为了达到该目的,通过在乙二醇中通过阳极氧化法制备了在Ti板上作为光阳极的高效纳米管结构的TiO 2,并通过XRD,SEM和光电流技术对其进行了表征。研究了纳米管的形貌,施加的电势,Na 2 SO 4的浓度,溶液的搅拌速度和pH对反应活性和产物选择性的影响。TiO 2所有阳极的相主要是锐钛矿。通过增加纳米管长度,PEC活性,XRD峰强度和光电流增加。活性通过同时降低Na 2 SO 4浓度和施加的电位而降低,而3-吡啶甲烷甲基选择性提高。通过提高溶液的搅拌速度,活性和3-吡啶甲醛的选择性均增加。为了进行比较,分别对光催化(PC)和电催化运行观察到较低或没有活性。在N 2存在下没有获得PC活性,但是在N 2存在下的PEC反应比在O 2存在下的PEC反应要快。。在PEC反应中,在N 2和O 2气氛中在阴极生成的3-吡啶甲烷甲醛均被还原,但其氧化似乎更为有利。PC反应不能在酸性条件下进行,而PEC反应可以在2-12的pH范围内进行。而且,结果表明,相对于文献报道的那些,PEC方法允许在pH 7下获得更高的转化率和对维生素B 3的选择性。
更新日期:2019-11-11
中文翻译:

TiO2纳米管将3-吡啶甲醇光催化氧化为3-吡啶甲醛和维生素B3
本文研究了第一个光电催化(PEC)3-吡啶甲醇氧化为3-吡啶甲烷甲醛和维生素B 3的方法。为了达到该目的,通过在乙二醇中通过阳极氧化法制备了在Ti板上作为光阳极的高效纳米管结构的TiO 2,并通过XRD,SEM和光电流技术对其进行了表征。研究了纳米管的形貌,施加的电势,Na 2 SO 4的浓度,溶液的搅拌速度和pH对反应活性和产物选择性的影响。TiO 2所有阳极的相主要是锐钛矿。通过增加纳米管长度,PEC活性,XRD峰强度和光电流增加。活性通过同时降低Na 2 SO 4浓度和施加的电位而降低,而3-吡啶甲烷甲基选择性提高。通过提高溶液的搅拌速度,活性和3-吡啶甲醛的选择性均增加。为了进行比较,分别对光催化(PC)和电催化运行观察到较低或没有活性。在N 2存在下没有获得PC活性,但是在N 2存在下的PEC反应比在O 2存在下的PEC反应要快。。在PEC反应中,在N 2和O 2气氛中在阴极生成的3-吡啶甲烷甲醛均被还原,但其氧化似乎更为有利。PC反应不能在酸性条件下进行,而PEC反应可以在2-12的pH范围内进行。而且,结果表明,相对于文献报道的那些,PEC方法允许在pH 7下获得更高的转化率和对维生素B 3的选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号