当前位置:
X-MOL 学术
›
Vib. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Room Temperature Gas Phase Infrared Spectra of H-bonded Oligomers of Methanol
Vibrational Spectroscopy ( IF 2.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.vibspec.2019.102981 Bedabyas Behera , Shubhadip Chakraborty
Vibrational Spectroscopy ( IF 2.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.vibspec.2019.102981 Bedabyas Behera , Shubhadip Chakraborty
|
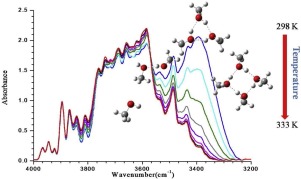
Abstract Formation of various hydrogen bonded oligomers of methanol at room temperature was investigated with the aid of gas phase infrared spectroscopy. In parallel quantum chemical vibrational analyses were performed to identify them and calculate their thermodymical properties. Above 20 torr several weak features appear to the lower wavenumber of the O-H stretch fundamental. Comparing with previous literature reports we ascribe them due to higher (di, tri and tetrameric) oligomeric forms of methanol. Increasing temperature above 298 K in an isochoric condition diminishes the intensity of the observed oligomeric bands, which provides the basis for identification of these bands as originating from H-bonded oligomers. Open and cyclic forms of di, tri and tetrameric forms of methanol were optimized and their anharmonic frequencies were calculated using second order vibrational perturbation theory. Additionally, their binding energies and the enthalpy of formations were also derived from the thermochemical analysis. Experimental enthalpy of formations were evaluated from the variation of integrated band areas with temperature. From the calculated oscillator strengths and the area under each band, the partial pressures of different H-bonded methanol oligomers at room temperature have been obtained for the first time. We obtained in 100 torr total pressure at 298 K the partial pressures of dimer, trimer and tetramer are 0.005, 0.0122 and 0.076 torr, respectively.
中文翻译:

甲醇氢键低聚物的室温气相红外光谱
摘要 借助气相红外光谱研究了室温下甲醇的各种氢键低聚物的形成。同时进行量子化学振动分析以识别它们并计算它们的热力学特性。在 20 托以上,OH 拉伸基本波的较低波数出现了几个弱特征。与以前的文献报道相比,我们将它们归因于甲醇的更高(二聚、三聚和四聚)低聚形式。在等容条件下将温度升高到 298 K 以上会降低观察到的寡聚带的强度,这为识别这些带源自 H 键合的寡聚体提供了基础。di 的开环形式,优化了三聚体和四聚体形式的甲醇,并使用二阶振动微扰理论计算了它们的非谐频率。此外,它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。首次获得了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。首次获得了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。
更新日期:2020-01-01
中文翻译:

甲醇氢键低聚物的室温气相红外光谱
摘要 借助气相红外光谱研究了室温下甲醇的各种氢键低聚物的形成。同时进行量子化学振动分析以识别它们并计算它们的热力学特性。在 20 托以上,OH 拉伸基本波的较低波数出现了几个弱特征。与以前的文献报道相比,我们将它们归因于甲醇的更高(二聚、三聚和四聚)低聚形式。在等容条件下将温度升高到 298 K 以上会降低观察到的寡聚带的强度,这为识别这些带源自 H 键合的寡聚体提供了基础。di 的开环形式,优化了三聚体和四聚体形式的甲醇,并使用二阶振动微扰理论计算了它们的非谐频率。此外,它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。它们的结合能和形成焓也来自热化学分析。从积分带面积随温度的变化来评估地层的实验焓。根据计算的振子强度和各带下面积,首次得到了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。首次获得了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。首次获得了不同氢键的甲醇低聚物在室温下的分压。我们在 298 K 和 100 托的总压力下获得二聚体、三聚体和四聚体的分压分别为 0.005、0.0122 和 0.076 托。



























 京公网安备 11010802027423号
京公网安备 11010802027423号