当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hepatitis C Virus Helicase Binding Activity Monitored through Site-Specific Labeling Using an Expanded Genetic Code.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2019-11-07 , DOI: 10.1021/acsinfecdis.9b00220 Christopher J Ablenas 1, 2 , Yasser Gidi 3 , Megan H Powdrill 2 , Noreen Ahmed 2 , Tyler A Shaw 2 , Mihai Mesko 3 , Matthias Götte 4 , Gonzalo Cosa 3 , John Paul Pezacki 2
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2019-11-07 , DOI: 10.1021/acsinfecdis.9b00220 Christopher J Ablenas 1, 2 , Yasser Gidi 3 , Megan H Powdrill 2 , Noreen Ahmed 2 , Tyler A Shaw 2 , Mihai Mesko 3 , Matthias Götte 4 , Gonzalo Cosa 3 , John Paul Pezacki 2
Affiliation
|
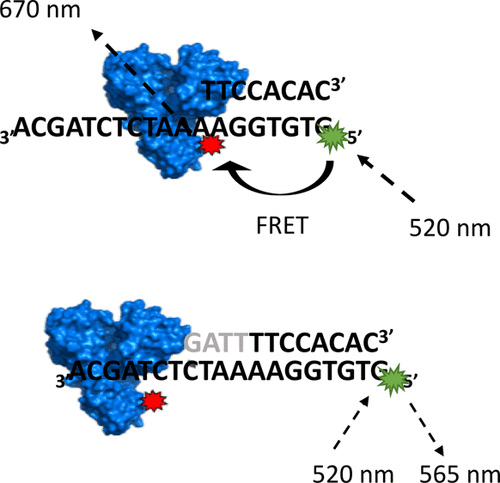
The mechanism of unwinding catalyzed by the hepatitis C virus nonstructural protein 3 helicase (NS3h) has been a subject of considerable interest, with NS3h serving as a prototypical enzyme in the study of helicase function. Recent studies support an ATP-fueled, inchworm-like stepping of NS3h on the nucleic acid that would result in the displacement of the complementary strand of the duplex during unwinding. Here, we describe the screening of a site of incorporation of an unnatural amino acid in NS3h for fluorescent labeling of the enzyme to be used in single-molecule Förster resonance energy transfer (FRET) experiments. From the nine potential sites identified in NS3h for incorporation of the unnatural amino acid, only one allowed for expression and fluorescent labeling of the recombinant protein. Incorporation of the unnatural amino acid was confirmed via bulk assays to not interfere with unwinding activity of the helicase. Binding to four different dsDNA sequences bearing a ssDNA overhang segment of varying length (either minimal 6 or 7 base length overhang to ensure binding or a long 24 base overhang) and sequence was recorded with the new NS3h construct at the single-molecule level. Single-molecule fluorescence displayed time intervals with anticorrelated donor and acceptor emission fluctuations associated with protein binding to the substrates. An apparent FRET value was estimated from the binding events showing a single FRET value of ∼0.8 for the 6–7 base overhangs. A smaller mean value and a broad distribution was in turn recorded for the long ssDNA overhang, consistent with NS3h exploring a larger physical space while bound to the DNA construct. Notably, intervals where NS3h binding was recorded were exhibited at time periods where the acceptor dye reversibly bleached. Protein induced fluorescence intensity enhancement in the donor channel became apparent at these intervals. Overall, the site-specific fluorescent labeling of NS3h reported here provides a powerful tool for future studies to monitor the dynamics of enzyme translocation during unwinding by single-molecule FRET.
中文翻译:

丙型肝炎病毒解旋酶结合活性通过使用扩展的遗传密码的特定于位点的标记进行监控。
丙型肝炎病毒非结构蛋白3解旋酶(NS3h)催化的放松机制已引起广泛关注,NS3h作为研究解旋酶功能的原型酶。最近的研究支持在核苷上以ATP刺激的尺inch样NS3h进入核酸,这将导致解链过程中双链体的互补链发生置换。在这里,我们描述了在NS3h中非天然氨基酸掺入位点的筛选,用于荧光标记用于单分子Förster共振能量转移(FRET)实验的酶。在NS3h中鉴定出的用于掺入非天然氨基酸的九个潜在位点中,仅一个位点允许重组蛋白的表达和荧光标记。通过大量测定证实了非天然氨基酸的掺入不干扰解旋酶的解旋活性。与带有不同长度ssDNA突出端片段的四个不同dsDNA序列的结合(最小6或7个碱基长度的突出端,以确保结合或长24碱基突出),并使用新的NS3h构建体以单分子水平记录序列。单分子荧光显示的时间间隔具有与蛋白质与底物结合相关的抗相关的供体和受体发射波动。由结合事件估计的表观FRET值表明6-7个碱基突出端的单个FRET值为〜0.8。反过来,对于较长的ssDNA突出端,记录的平均值较小,分布较宽,与NS3h一致,在与DNA构建体结合的同时探索更大的物理空间。值得注意的是,在受体染料可逆地漂白的时间段,记录了记录NS3h结合的间隔。在这些间隔下,供体通道中蛋白质诱导的荧光强度增强变得明显。总体而言,此处报道的NS3h的位点特异性荧光标记为将来的研究提供了强大的工具,以监控单分子FRET展开过程中酶转运的动力学。
更新日期:2019-11-08
中文翻译:

丙型肝炎病毒解旋酶结合活性通过使用扩展的遗传密码的特定于位点的标记进行监控。
丙型肝炎病毒非结构蛋白3解旋酶(NS3h)催化的放松机制已引起广泛关注,NS3h作为研究解旋酶功能的原型酶。最近的研究支持在核苷上以ATP刺激的尺inch样NS3h进入核酸,这将导致解链过程中双链体的互补链发生置换。在这里,我们描述了在NS3h中非天然氨基酸掺入位点的筛选,用于荧光标记用于单分子Förster共振能量转移(FRET)实验的酶。在NS3h中鉴定出的用于掺入非天然氨基酸的九个潜在位点中,仅一个位点允许重组蛋白的表达和荧光标记。通过大量测定证实了非天然氨基酸的掺入不干扰解旋酶的解旋活性。与带有不同长度ssDNA突出端片段的四个不同dsDNA序列的结合(最小6或7个碱基长度的突出端,以确保结合或长24碱基突出),并使用新的NS3h构建体以单分子水平记录序列。单分子荧光显示的时间间隔具有与蛋白质与底物结合相关的抗相关的供体和受体发射波动。由结合事件估计的表观FRET值表明6-7个碱基突出端的单个FRET值为〜0.8。反过来,对于较长的ssDNA突出端,记录的平均值较小,分布较宽,与NS3h一致,在与DNA构建体结合的同时探索更大的物理空间。值得注意的是,在受体染料可逆地漂白的时间段,记录了记录NS3h结合的间隔。在这些间隔下,供体通道中蛋白质诱导的荧光强度增强变得明显。总体而言,此处报道的NS3h的位点特异性荧光标记为将来的研究提供了强大的工具,以监控单分子FRET展开过程中酶转运的动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号