当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Real-Time BODIPY-Binding Assay To Screen Inhibitors of the Early Oligomerization Process of Aβ1-42 Peptide.
ChemBioChem ( IF 3.2 ) Pub Date : 2020-01-09 , DOI: 10.1002/cbic.201900652 Nicolo Tonali 1, 2 , Veronica I Dodero 1 , Julia Kaffy 2 , Loreen Hericks 1 , Sandrine Ongeri 2 , Norbert Sewald 1
ChemBioChem ( IF 3.2 ) Pub Date : 2020-01-09 , DOI: 10.1002/cbic.201900652 Nicolo Tonali 1, 2 , Veronica I Dodero 1 , Julia Kaffy 2 , Loreen Hericks 1 , Sandrine Ongeri 2 , Norbert Sewald 1
Affiliation
|
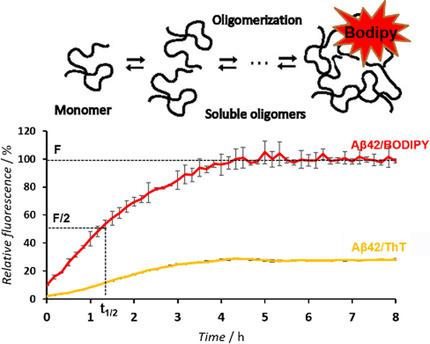
Misfolding and aggregation of amyloid β1-42 peptide (Aβ1-42) play a central role in the pathogenesis of Alzheimer's disease (AD). Targeting the highly cytotoxic oligomeric species formed during the early stages of the aggregation process represents a promising therapeutic strategy to reduce the toxicity associated with Aβ1-42. Currently, the thioflavin T (ThT) assay is the only established spectrofluorometric method to screen aggregation inhibitors. The success of the ThT assay is that it can detect Aβ1-42 aggregates with high β-sheet content, such as protofibrils or fibrils, which appear in the late aggregation steps. Unfortunately, by using the ThT assay, the detection of inhibitors of early soluble oligomers that present a low β-sheet character is challenging. Herein, a new, facile, and robust boron-dipyrromethene (BODIPY) real-time assay suitable for 96-well plate format, which allows screening of compounds as selective inhibitors of the formation of Aβ1-42 oligomers, is reported. These inhibitors decrease the cellular toxicity of Aβ1-42, although they fail in the ThT assay. The findings have been confirmed and validated by structural analysis and cell viability assays under comparable experimental conditions. It is demonstrated that the BODIPY assay is a convenient method to screen and discover new candidate compounds that slow down or stop the pathological early oligomerization process and are active in the cellular assay. Therefore, it is a suitable complementary screening method of the current ThT assay.
中文翻译:

实时BODIPY结合试验可筛选Aβ1-42肽早期寡聚过程的抑制剂。
淀粉样蛋白β1-42肽(Aβ1-42)的错误折叠和聚集在阿尔茨海默氏病(AD)的发病机理中起着核心作用。靶向在聚集过程的早期形成的高度细胞毒性的寡聚体代表了减少与Aβ1-42相关的毒性的有前途的治疗策略。当前,硫黄素T(ThT)测定法是建立聚集抑制剂的唯一建立的分光荧光法。ThT分析的成功之处在于它可以检测到具有高β-sheet含量的Aβ1-42聚集体,例如原纤维或原纤维,这些聚集体出现在聚集后期。不幸的是,通过使用ThT分析,检测具有低β-折叠特性的早期可溶性低聚物的抑制剂具有挑战性。在这里,一种新的,便捷的,报道了适用于96孔板形式的稳健的硼二吡咯亚甲基(BODIPY)实时测定法,该方法可筛选作为Aβ1-42低聚物形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或停止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。据报道,其可以筛选作为Aβ1-42寡聚体形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。据报道,其可以筛选作为Aβ1-42寡聚体形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。在可比的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。
更新日期:2020-01-09
中文翻译:

实时BODIPY结合试验可筛选Aβ1-42肽早期寡聚过程的抑制剂。
淀粉样蛋白β1-42肽(Aβ1-42)的错误折叠和聚集在阿尔茨海默氏病(AD)的发病机理中起着核心作用。靶向在聚集过程的早期形成的高度细胞毒性的寡聚体代表了减少与Aβ1-42相关的毒性的有前途的治疗策略。当前,硫黄素T(ThT)测定法是建立聚集抑制剂的唯一建立的分光荧光法。ThT分析的成功之处在于它可以检测到具有高β-sheet含量的Aβ1-42聚集体,例如原纤维或原纤维,这些聚集体出现在聚集后期。不幸的是,通过使用ThT分析,检测具有低β-折叠特性的早期可溶性低聚物的抑制剂具有挑战性。在这里,一种新的,便捷的,报道了适用于96孔板形式的稳健的硼二吡咯亚甲基(BODIPY)实时测定法,该方法可筛选作为Aβ1-42低聚物形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或停止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。据报道,其可以筛选作为Aβ1-42寡聚体形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。据报道,其可以筛选作为Aβ1-42寡聚体形成的选择性抑制剂的化合物。尽管这些抑制剂在ThT分析中失败,但它们降低了Aβ1-42的细胞毒性。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。在可比的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。在相当的实验条件下,通过结构分析和细胞生存力测定法证实并证实了这一发现。已证明,BODIPY测定法是筛选和发现新的候选化合物的便捷方法,这些候选化合物可减慢或终止病理性早期寡聚过程并在细胞测定法中具有活性。因此,它是当前ThT分析的一种合适的补充筛选方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号