Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Basis for the PZP Domain of BRPF1 Association with Chromatin.
Structure ( IF 5.7 ) Pub Date : 2019-11-08 , DOI: 10.1016/j.str.2019.10.014 Brianna J Klein 1 , Khan L Cox 2 , Suk Min Jang 3 , Jacques Côté 3 , Michael G Poirier 2 , Tatiana G Kutateladze 1
Structure ( IF 5.7 ) Pub Date : 2019-11-08 , DOI: 10.1016/j.str.2019.10.014 Brianna J Klein 1 , Khan L Cox 2 , Suk Min Jang 3 , Jacques Côté 3 , Michael G Poirier 2 , Tatiana G Kutateladze 1
Affiliation
|
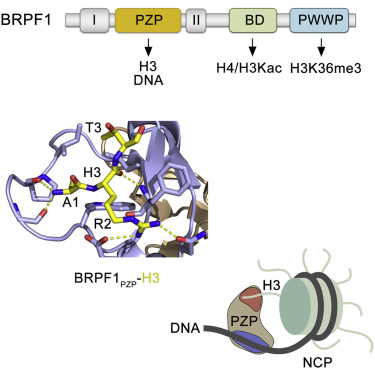
The assembly of human histone acetyltransferase MOZ/MORF complexes relies on the scaffolding bromodomain plant homeodomain (PHD) finger 1 (BRPF1) subunit. The PHD-zinc-knuckle-PHD module of BRPF1 (BRPF1PZP) has been shown to associate with the histone H3 tail and DNA; however, the molecular mechanism underlying recognition of H3 and the relationship between the histone and DNA-binding activities remain unclear. In this study, we report the crystal structure of BRPF1PZP bound to the H3 tail and characterize the role of the bipartite interaction in the engagement of BRPF1PZP with the nucleosome core particle (NCP). We find that although both interactions of BRPF1PZP with the H3 tail and DNA are required for tight binding to NCP and for acetyltransferase function of the BRPF1-MORF-ING5-MEAF6 complex, binding to extranucleosomal DNA dominates. Our findings suggest that functionally active BRPF1PZP might be important in stabilization of the MOZ/MORF complexes at chromatin with accessible DNA.
中文翻译:

BRPF1与染色质缔合的PZP域的分子基础。
人类组蛋白乙酰转移酶MOZ / MORF复合物的组装依赖于脚手架溴域植物同源域(PHD)手指1(BRPF1)亚基。已显示BRPF1(BRPF1PZP)的PHD-锌-指-PHD模块与组蛋白H3尾巴和DNA相关。然而,尚不清楚H3识别的潜在分子机制以及组蛋白与DNA结合活性之间的关系。在这项研究中,我们报告绑定到H3尾巴的BRPF1PZP的晶体结构,并表征了二方相互作用在BRPF1PZP与核小体核心粒子(NCP)结合中的作用。我们发现,尽管BRPF1PZP与H3尾巴和DNA的相互作用都是紧密结合NCP和BRPF1-MORF-ING5-MEAF6复合体的乙酰转移酶功能所必需的,但与核小体DNA的结合仍占主导地位。
更新日期:2019-11-08
中文翻译:

BRPF1与染色质缔合的PZP域的分子基础。
人类组蛋白乙酰转移酶MOZ / MORF复合物的组装依赖于脚手架溴域植物同源域(PHD)手指1(BRPF1)亚基。已显示BRPF1(BRPF1PZP)的PHD-锌-指-PHD模块与组蛋白H3尾巴和DNA相关。然而,尚不清楚H3识别的潜在分子机制以及组蛋白与DNA结合活性之间的关系。在这项研究中,我们报告绑定到H3尾巴的BRPF1PZP的晶体结构,并表征了二方相互作用在BRPF1PZP与核小体核心粒子(NCP)结合中的作用。我们发现,尽管BRPF1PZP与H3尾巴和DNA的相互作用都是紧密结合NCP和BRPF1-MORF-ING5-MEAF6复合体的乙酰转移酶功能所必需的,但与核小体DNA的结合仍占主导地位。


























 京公网安备 11010802027423号
京公网安备 11010802027423号