JAMA Oncology ( IF 28.4 ) Pub Date : 2020-01-01 , DOI: 10.1001/jamaoncol.2019.3848 Kimberley M Heinhuis 1 , Matteo Carlino 2 , Markus Joerger 3 , Massimo Di Nicola 4 , Tarek Meniawy 5 , Sylvie Rottey 6 , Victor Moreno 7 , Anas Gazzah 8 , Jean-Pierre Delord 9 , Luis Paz-Ares 10 , Christian Britschgi 11 , Russell J Schilder 12 , Kenneth O'Byrne 13 , Giuseppe Curigliano 14 , Emanuela Romano 15 , Poliana Patah 16 , Rui Wang 16 , Yali Liu 16 , Gaurav Bajaj 16 , Lillian L Siu 17
|
Importance Multiple immunostimulatory agonist antibodies have been clinically tested in solid tumors to evaluate the role of targeting glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein in anticancer treatments.
Objective To evaluate the safety and activity of the fully human glucocorticoid-induced TNF receptor–related protein agonist IgG1 monoclonal antibody BMS-986156 with or without nivolumab in patients with advanced solid tumors.
Design, Setting, and Participants This global, open-label, phase 1/2a study of BMS-986156 with or without nivolumab enrolled 292 patients 18 years or older with advanced solid tumors and an Eastern Cooperative Oncology Group performance status of 1 or less. Prior checkpoint inhibitor therapy was allowed. Monotherapy and combination dose-escalation cohorts ran concurrently to guide expansion doses beginning October 16, 2015; the study is ongoing.
Interventions The protein agonist BMS-986156 was administered intravenously at a dose of 10, 30, 100, 240, or 800 mg every 2 weeks as monotherapy, and in the combination group 30, 100, 240, or 800 mg plus 240 mg of nivolumab every 2 weeks; same-dose cohorts were pooled for analysis. One cohort also received 480 mg of BMS-986156 plus 480 mg of nivolumab every 4 weeks.
Main Outcomes and Measures The primary end points were safety, tolerability, and dose-limiting toxic effects. Additional end points included antitumor activity per Response Evaluation Criteria in Solid Tumors, version 1.1, and exploratory biomarker analyses.
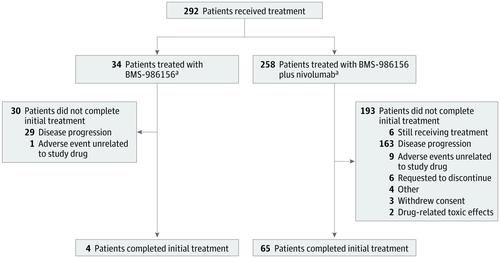
Results With a follow-up range of 1.4 to 101.7 weeks (follow-up ongoing), 34 patients (16 women and 18 men; median age, 56.6 years [range, 28-75 years]) received monotherapy (4 patients completed initial treatment), and 258 patients (140 women and 118 men; median age, 60 years [range, 21-87 years]) received combination therapy (65 patients completed initial treatment). No grade 3 to 5 treatment-related adverse events occurred with BMS-986156 monotherapy; grade 3 to 4 treatment-related adverse events occurred in 24 patients (9.3%) receiving BMS-986156 plus nivolumab, with no grade 5 treatment-related adverse events. One dose-limiting toxic effect (grade 4 elevated creatine phosphokinase levels) occurred in a patient receiving 800 mg of BMS-986156 plus 240 mg of nivolumab every 2 weeks; BMS-986156 with or without nivolumab exhibited linear pharmacokinetics with dose-related increase after a single dose. Peripheral T-cell and natural killer–cell proliferation increased after administration of BMS-986156 with or without nivolumab. No consistent and significant modulation of intratumoral CD8+ T cells and FoxP3+ regulatory T cells was observed. No responses were seen with BMS-986156 alone; objective response rates ranged from 0% to 11.1% (1 of 9) across combination therapy cohorts, with a few responses observed in patients previously treated with anti–programmed death receptor (ligand) 1 therapy.
Conclusions and Relevance Based on this cohort, BMS-986156 appears to have had a manageable safety profile, and BMS-986156 plus nivolumab demonstrated safety and efficacy comparable to historical data reported for nivolumab monotherapy.
Trial Registration ClinicalTrials.gov identifier: NCT02598960
中文翻译:

糖皮质激素诱导的 TNF 受体相关蛋白激动剂单独或与 Nivolumab 联合用于晚期实体瘤患者的安全性、耐受性和潜在临床活性:1/2a 期剂量递增和队列扩展临床试验
重要性 多种免疫刺激激动剂抗体已在实体瘤中进行了临床测试,以评估靶向糖皮质激素诱导的肿瘤坏死因子 (TNF) 受体相关蛋白在抗癌治疗中的作用。
目的 评估全人糖皮质激素诱导的 TNF 受体相关蛋白激动剂 IgG1 单克隆抗体 BMS-986156 联合或不联合纳武单抗在晚期实体瘤患者中的安全性和活性。
设计、设置和参与者 这项全球性、开放标签、1/2a 期 BMS-986156 研究纳入了 292 名 18 岁或以上患有晚期实体瘤且东部肿瘤协作组体能状态为 1 或更低的患者。允许先前的检查点抑制剂治疗。从 2015 年 10 月 16 日开始,单药治疗和联合剂量递增队列同时进行以指导扩展剂量;该研究正在进行中。
干预 蛋白激动剂 BMS-986156 以每 2 周 10、30、100、240 或 800 mg 的剂量静脉内给药,作为单一疗法,在联合组中 30、100、240 或 800 mg 加 240 mg 纳武利尤单抗每两周一次;合并相同剂量的队列进行分析。一个队列还每 4 周接受 480 mg BMS-986156 和 480 mg nivolumab。
主要结果和措施 主要终点是安全性、耐受性和剂量限制性毒性作用。其他终点包括根据实体瘤反应评估标准 1.1 版和探索性生物标志物分析的抗肿瘤活性。
结果 在 1.4 至 101.7 周的随访范围内(随访进行中),34 名患者(16 名女性和 18 名男性;中位年龄,56.6 岁 [范围,28-75 岁])接受了单药治疗(4 名患者完成了初始治疗) ,以及 258 名患者(140 名女性和 118 名男性;中位年龄,60 岁 [范围,21-87 岁])接受了联合治疗(65 名患者完成了初始治疗)。BMS-986156 单药治疗未发生 3 至 5 级治疗相关不良事件;接受 BMS-986156 加 nivolumab 的 24 名患者 (9.3%) 发生 3 至 4 级治疗相关不良事件,没有 5 级治疗相关不良事件。一名接受 800 mg BMS-986156 加 240 mg nivolumab 每 2 周的患者发生了一种剂量限制性毒性作用(4 级肌酸磷酸激酶水平升高);BMS-986156 加或不加 nivolumab 表现出线性药代动力学,单次给药后剂量相关增加。BMS-986156 联合或不联合纳武利尤单抗后,外周 T 细胞和自然杀伤细胞增殖增加。瘤内 CD8 没有一致和显着的调节观察到+ T 细胞和 FoxP3 +调节性 T 细胞。单独使用 BMS-986156 没有反应;在联合治疗队列中,客观缓解率从 0% 到 11.1%(9 人中的 1 人)不等,在先前接受过抗程序性死亡受体(配体)1 治疗的患者中观察到一些反应。
结论和相关性 基于该队列,BMS-986156 似乎具有可控的安全性,BMS-986156 加 nivolumab 的安全性和有效性可与 nivolumab 单药治疗的历史数据相媲美。
试验注册 ClinicalTrials.gov 标识符:NCT02598960


























 京公网安备 11010802027423号
京公网安备 11010802027423号