当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma.
Biomaterials Science ( IF 6.6 ) Pub Date : 2019-11-12 , DOI: 10.1039/c9bm01766f Roberto Molinaro 1 , Jonathan O Martinez 2 , Assaf Zinger 2 , Alessandro De Vita 3 , Gianluca Storci 4 , Noemi Arrighetti 5 , Enrica De Rosa 2 , Kelly A Hartman 2 , Nupur Basu 2 , Nima Taghipour 2 , Claudia Corbo 6 , Ennio Tasciotti 7
Biomaterials Science ( IF 6.6 ) Pub Date : 2019-11-12 , DOI: 10.1039/c9bm01766f Roberto Molinaro 1 , Jonathan O Martinez 2 , Assaf Zinger 2 , Alessandro De Vita 3 , Gianluca Storci 4 , Noemi Arrighetti 5 , Enrica De Rosa 2 , Kelly A Hartman 2 , Nupur Basu 2 , Nima Taghipour 2 , Claudia Corbo 6 , Ennio Tasciotti 7
Affiliation
|
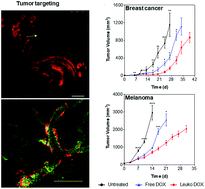
In the last decades, several approaches were developed to design drug delivery systems to address the multiple biological barriers encountered after administration while safely delivering a payload. In this scenario, bio-inspired and bio-mimetic approaches have emerged as promising solutions to evade the mononuclear phagocytic system while simultaneously negotiating the sequential transport across the various biological barriers. Leukocytes freely circulate in the bloodstream and selectively target the inflamed vasculature in response to injury, infection, and cancer. Recently we have shown the use of biomimetic nanovesicles, called leukosomes, which combine both the physical and biological properties of liposomes and leukocytes, respectively, to selectively deliver drugs to the inflamed vasculature. Here we report the use of leukosomes to target and deliver doxorubicin, a model chemotherapeutic, to tumors in syngeneic murine models of breast cancer and melanoma. Exploiting the inflammatory pathway responsible for recruiting immune cells to the site of injury, leukosomes exhibited increased targeting of cancer vasculature and stroma. Furthermore, delivery of doxorubicin with leukosomes enabled significant tumor growth inhibition compared with free doxorubicin in both breast and melanoma tumors. This study demonstrates the promise of using biomimetic nanovesicles for effective cancer management in solid tumors.
中文翻译:

模仿白细胞的纳米囊泡,有效递送阿霉素以治疗乳腺癌和黑色素瘤。
在过去的几十年中,开发了几种方法来设计药物输送系统,以解决给药后遇到的多种生物障碍,同时安全地输送有效载荷。在这种情况下,以生物启发和仿生生物的方法已经成为逃避单核吞噬系统的有希望的解决方案,同时又谈判了跨越各种生物屏障的顺序运输。白细胞在血液中自由循环并响应损伤,感染和癌症而选择性靶向发炎的脉管系统。最近,我们显示了仿生纳米囊泡(称为白细胞体)的使用,它们分别结合了脂质体和白细胞的物理和生物学特性,以选择性地将药物递送至发炎的脉管系统。在这里,我们报道了使用白细胞靶向和递送阿霉素(一种模型化学疗法)到乳腺癌和黑色素瘤的同基因鼠模型中的肿瘤。利用负责将免疫细胞募集到损伤部位的炎症途径,白细胞表现出对癌症脉管系统和基质的靶向增加。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。白细胞显示出增加的针对癌症脉管系统和间质的靶向性。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。白细胞显示出增加的针对癌症脉管系统和间质的靶向性。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。
更新日期:2019-12-18
中文翻译:

模仿白细胞的纳米囊泡,有效递送阿霉素以治疗乳腺癌和黑色素瘤。
在过去的几十年中,开发了几种方法来设计药物输送系统,以解决给药后遇到的多种生物障碍,同时安全地输送有效载荷。在这种情况下,以生物启发和仿生生物的方法已经成为逃避单核吞噬系统的有希望的解决方案,同时又谈判了跨越各种生物屏障的顺序运输。白细胞在血液中自由循环并响应损伤,感染和癌症而选择性靶向发炎的脉管系统。最近,我们显示了仿生纳米囊泡(称为白细胞体)的使用,它们分别结合了脂质体和白细胞的物理和生物学特性,以选择性地将药物递送至发炎的脉管系统。在这里,我们报道了使用白细胞靶向和递送阿霉素(一种模型化学疗法)到乳腺癌和黑色素瘤的同基因鼠模型中的肿瘤。利用负责将免疫细胞募集到损伤部位的炎症途径,白细胞表现出对癌症脉管系统和基质的靶向增加。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。白细胞显示出增加的针对癌症脉管系统和间质的靶向性。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。白细胞显示出增加的针对癌症脉管系统和间质的靶向性。此外,与游离阿霉素相比,在乳腺和黑素瘤肿瘤中,将阿霉素与白体一起递送能够显着抑制肿瘤生长。这项研究证明了使用仿生纳米囊泡在实体瘤中有效治疗癌症的前景。


























 京公网安备 11010802027423号
京公网安备 11010802027423号