当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
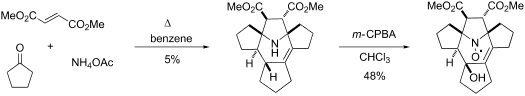
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-11-07 , DOI: 10.3762/bjoc.15.259 Sergey A Dobrynin , Igor A Kirilyuk , Yuri V Gatilov , Andrey A Kuzhelev , Olesya A Krumkacheva , Matvey V Fedin , Michael K Bowman , Elena G Bagryanskaya
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2019-11-07 , DOI: 10.3762/bjoc.15.259 Sergey A Dobrynin , Igor A Kirilyuk , Yuri V Gatilov , Andrey A Kuzhelev , Olesya A Krumkacheva , Matvey V Fedin , Michael K Bowman , Elena G Bagryanskaya
|
The unexpected formation of a highly strained polycyclic amine was observed in a one-pot synthesis from cyclopentanone, dimethyl fumarate and ammonium acetate. This multistep reaction includes 1,3-dipolar cycloaddition of dimethyl fumarate to the cyclic azomethine ylide formed in situ from cyclopentanone and ammonia. The polycyclic amine product was easily converted into a sterically shielded polycyclic nitroxide. The EPR spectra and spin relaxation behavior of the nitroxide were studied in solution. The spin relaxation seems well suited for the use as a biological spin label and are comparable with those of cyclic nitroxides with two spirocyclic moieties adjacent to the N–O· group.
中文翻译:

由环戊酮,氨和富马酸二甲酯意外地一锅生成1H-6a,8a-表三三环戊[a,c,e] [8]环戊烯体系。高应变多环氮氧化物的合成及EPR研究
在由一环戊酮,富马酸二甲酯和乙酸铵进行的一锅法合成中观察到了高应变多环胺的意外形成。该多步骤反应包括将富马酸二甲酯的1,3-偶极环加成到由环戊酮和氨原位形成的环状偶氮甲叶立德。多环胺产物易于转化为空间屏蔽的多环氮氧化物。研究了溶液中氮氧化物的EPR谱和自旋弛豫行为。自旋弛豫似乎非常适合用作生物自旋标记,并且与具有两个邻近N–O ·基团的螺环部分的环状氮氧化物的自旋弛豫相当。
更新日期:2019-11-07
中文翻译:

由环戊酮,氨和富马酸二甲酯意外地一锅生成1H-6a,8a-表三三环戊[a,c,e] [8]环戊烯体系。高应变多环氮氧化物的合成及EPR研究
在由一环戊酮,富马酸二甲酯和乙酸铵进行的一锅法合成中观察到了高应变多环胺的意外形成。该多步骤反应包括将富马酸二甲酯的1,3-偶极环加成到由环戊酮和氨原位形成的环状偶氮甲叶立德。多环胺产物易于转化为空间屏蔽的多环氮氧化物。研究了溶液中氮氧化物的EPR谱和自旋弛豫行为。自旋弛豫似乎非常适合用作生物自旋标记,并且与具有两个邻近N–O ·基团的螺环部分的环状氮氧化物的自旋弛豫相当。



























 京公网安备 11010802027423号
京公网安备 11010802027423号