当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Free Energy Landscape To Predict the Surfactant Adsorption Isotherm at the Nanoparticle–Water Interface
ACS Central Science ( IF 18.2 ) Pub Date : 2019-11-05 , DOI: 10.1021/acscentsci.9b00773 Paolo De Angelis 1 , Annalisa Cardellini 1 , Pietro Asinari 1
ACS Central Science ( IF 18.2 ) Pub Date : 2019-11-05 , DOI: 10.1021/acscentsci.9b00773 Paolo De Angelis 1 , Annalisa Cardellini 1 , Pietro Asinari 1
Affiliation
|
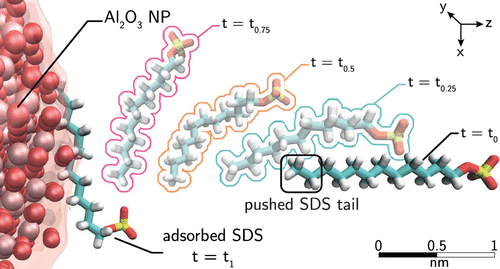
The long-lasting stability of nanoparticle (NP) suspensions in aqueous solution is one of the main challenges in colloidal science. The addition of surfactants is generally adopted to increase the free energy barrier between NPs and hence to ensure a more stable condition avoiding the NP sedimentation. However, a tailored prediction of surfactant concentration enabling a good dispersion of NPs is still an ambitious objective. Here, we demonstrate the efficiency of coupling steered molecular dynamics (SMD) with the Langmuir theory of adsorption in the low surfactant concentration regime, to predict the adsorption isotherm of sodium-dodecyl-sulfate (SDS) on bare α-alumina NPs suspended in aqueous solution. The resulting adsorption free energy landscapes (FELs) are also investigated by tuning the percentage of SDS molecules coating the target bare NP. Our findings shed light on the competing role of enthalpic and entropic interaction contributions. On one hand, the adsorption is highly promoted by the tail–NP and tail–tail nonbonded interaction adhesion; on the other hand, our results unveil the entropic nature of water and surfactant steric effects occurring at the NP surface and preventing the adsorption. Finally, a thorough analysis on the steering works emphasizes the role of the NP curvature in the FEL of adsorption. In particular, we show that, moving from a solid infinite flat surface to a nanoscale particle, a deviation from a Markovian dynamics of adsorption occurs in close proximity to a curved solid–liquid interface. Here, both the NP curvature effect and nanoscale morphology promote a modification of the thermodynamics state of adsorption with a consequent splitting of the free energy profiles and the identification of specific sites of adsorption. The modeling framework suggested in this Article provides physical insights in the surfactant adsorption onto spherical NPs and suggests some guidelines to rationally design stable NP suspensions in aqueous solutions.
中文翻译:

探索自由能格局,以预测表面活性剂在纳米颗粒-水界面的吸附等温线
纳米粒子(NP)悬浮液在水溶液中的持久稳定性是胶体科学的主要挑战之一。通常采用表面活性剂的添加来增加NP之间的自由能垒,从而确保避免NP沉降的更稳定的条件。但是,对表面活性剂浓度进行量身定制的预测能够实现NP的良好分散仍然是一个雄心勃勃的目标。在这里,我们证明了在低表面活性剂浓度条件下,利用Langmuir吸附理论耦合操纵分子动力学(SMD)的效率,可以预测十二烷基硫酸钠(SDS)在悬浮于水溶液中的裸露α-氧化铝纳米颗粒上的吸附等温线。解决方案。还通过调整覆盖目标裸NP的SDS分子的百分比来研究所得的吸附自由能态(FEL)。我们的发现揭示了焓和熵相互作用贡献的竞争作用。一方面,尾-NP和尾-尾非键相互作用的粘附力极大地促进了吸附。另一方面,我们的结果揭示了水的熵性质以及在NP表面发生的表面活性剂的空间位阻效应并阻止了吸附。最后,对转向工作的透彻分析强调了NP曲率在吸附FEL中的作用。尤其是,我们表明,从固体无限平坦的表面移动到纳米级粒子,会在非常接近弯曲的固液界面的地方发生偏离吸附的马尔可夫动力学的现象。这里,NP曲率效应和纳米级形态都促进了吸附的热力学状态的改变,其结果是自由能分布的分裂和对特定吸附位点的识别。本文中建议的建模框架提供了有关表面活性剂吸附到球形NP上的物理见解,并提出了一些指南,以合理设计水溶液中稳定的NP悬浮液。
更新日期:2019-11-28
中文翻译:

探索自由能格局,以预测表面活性剂在纳米颗粒-水界面的吸附等温线
纳米粒子(NP)悬浮液在水溶液中的持久稳定性是胶体科学的主要挑战之一。通常采用表面活性剂的添加来增加NP之间的自由能垒,从而确保避免NP沉降的更稳定的条件。但是,对表面活性剂浓度进行量身定制的预测能够实现NP的良好分散仍然是一个雄心勃勃的目标。在这里,我们证明了在低表面活性剂浓度条件下,利用Langmuir吸附理论耦合操纵分子动力学(SMD)的效率,可以预测十二烷基硫酸钠(SDS)在悬浮于水溶液中的裸露α-氧化铝纳米颗粒上的吸附等温线。解决方案。还通过调整覆盖目标裸NP的SDS分子的百分比来研究所得的吸附自由能态(FEL)。我们的发现揭示了焓和熵相互作用贡献的竞争作用。一方面,尾-NP和尾-尾非键相互作用的粘附力极大地促进了吸附。另一方面,我们的结果揭示了水的熵性质以及在NP表面发生的表面活性剂的空间位阻效应并阻止了吸附。最后,对转向工作的透彻分析强调了NP曲率在吸附FEL中的作用。尤其是,我们表明,从固体无限平坦的表面移动到纳米级粒子,会在非常接近弯曲的固液界面的地方发生偏离吸附的马尔可夫动力学的现象。这里,NP曲率效应和纳米级形态都促进了吸附的热力学状态的改变,其结果是自由能分布的分裂和对特定吸附位点的识别。本文中建议的建模框架提供了有关表面活性剂吸附到球形NP上的物理见解,并提出了一些指南,以合理设计水溶液中稳定的NP悬浮液。



























 京公网安备 11010802027423号
京公网安备 11010802027423号