Joule ( IF 39.8 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.joule.2019.10.006 Vasileios Kyriakou , Ioannis Garagounis , Anastasios Vourros , Eirini Vasileiou , Michael Stoukides
|
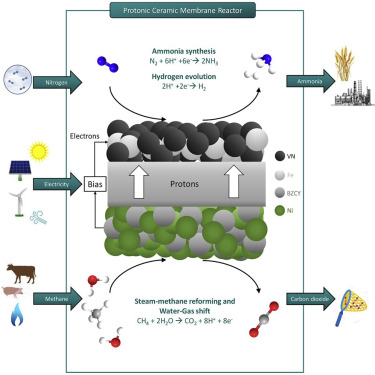
Ammonia, produced via the Haber-Bosch (HB) process, is globally the leading chemical in energy consumption and carbon dioxide emissions. In ammonia plants, hydrogen is generated by steam-methane reforming (SMR) and water-gas shift (WGS) and, subsequently, is purified for the high-pressure ammonia synthesis. Herein, we demonstrate how these steps are integrated into a single BaZrO3-based protonic ceramic membrane reactor (PCMR), operating at atmospheric pressure. Hydrogen generation occurs on a Ni-composite electrode, while VN-Fe is the ammonia synthesis electrocatalyst. Hydrogen extraction from the reforming compartment enhances the thermodynamically limited methane conversions, whereas 5%–14% of the pumped protons are converted to ammonia. An electrochemical HB is designed by combining this PCMR with a protonic ceramic fuel cell to recover electricity and separate nitrogen from ambient air by exploiting by-product hydrogen. This process could potentially require less energy and release less carbon dioxide emissions than its conventional counterpart, holding promise for sustainable decentralized applications.
中文翻译:

电化学Haber-Bosch工艺
通过哈伯-博世(HB)工艺生产的氨是能源消耗和二氧化碳排放方面的全球领先化学品。在氨厂中,通过蒸汽-甲烷重整(SMR)和水煤气变换(WGS)产生氢气,然后将其纯化以用于高压氨合成。本文中,我们演示了如何将这些步骤集成到单个BaZrO 3中质子的陶瓷质子膜反应器(PCMR),在大气压下运行。氢的生成发生在镍复合电极上,而VN-Fe是氨合成电催化剂。从重整室提取氢气可提高热力学上限制的甲烷转化率,而5%–14%的抽运质子会转化为氨。通过将这种PCMR与质子陶瓷燃料电池结合使用,可以设计电化学HB,以回收电并通过利用副产物氢来从环境空气中分离出氮。与传统工艺相比,此工艺可能需要更少的能源并释放出更少的二氧化碳,这为可持续的分散式应用带来了希望。


























 京公网安备 11010802027423号
京公网安备 11010802027423号