当前位置:
X-MOL 学术
›
Prostate Cancer Prostatic. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adverse events related to abiraterone and enzalutamide treatment: analysis of the EudraVigilance database and meta-analysis of registrational phase III studies.
Prostate Cancer and Prostatic Diseases ( IF 4.8 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41391-019-0182-x Cosimo De Nunzio 1 , Riccardo Lombardo 1 , Giorgia Tema 1 , Olivia Voglino 1 , Angela Sica 1 , Valeria Baldassarri 1 , Antonio Nacchia 1 , Roberto Iacovelli 2 , Sergio Bracarda 3 , Andrea Tubaro 1
Prostate Cancer and Prostatic Diseases ( IF 4.8 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41391-019-0182-x Cosimo De Nunzio 1 , Riccardo Lombardo 1 , Giorgia Tema 1 , Olivia Voglino 1 , Angela Sica 1 , Valeria Baldassarri 1 , Antonio Nacchia 1 , Roberto Iacovelli 2 , Sergio Bracarda 3 , Andrea Tubaro 1
Affiliation
|
BACKGROUND
Data from clinical trials do not always provide adequate information to judge the impact of new treatments when used in a real-world setting. The aim of our study was to analyze adverse events (AEs) associated with enzalutamide (ENZ) and abiraterone (ABI) using real-life data from the EudraVigilance (EV) database.
METHODS
The EV database is the system for managing and analyzing information on suspected adverse reactions to medicines, which have been authorized or are being studied in clinical trials in the European Economic Area. We recorded the number of AEs for ABI and ENZ per category and severity from January 2013 to January 2019. In addition, we recorded AEs per age group. A meta-analysis of AEs reported in registrational phase III studies (AFFIRM, PREVAIL, COU-AA) was performed.
RESULTS
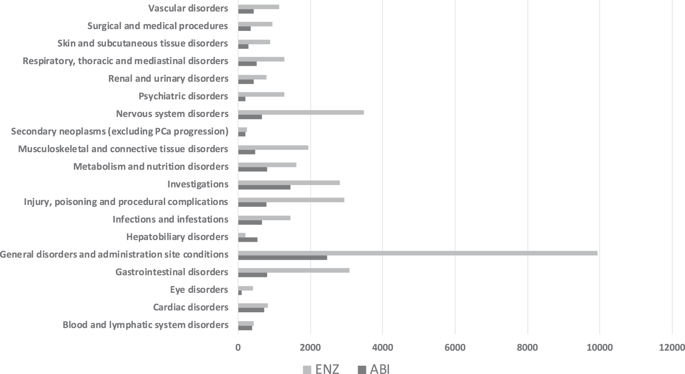
The number of individual cases identified in EV database was 13,562 for ABI and 40,599 for ENZ. Over 90% of the reported AEs were defined as serious for both drugs. Older patients (>85 years and 65-85 years) treated with ABI or ENZ are at increased risk of cardiac, infectious, metabolic, and respiratory disorders when compared with younger patients (<65). According to registrational phase III studies, the most frequent AEs in patients treated with ABI are hepatobiliary disorders, while the most frequent AEs in patients treated with ENZ are psychiatric and vascular disorders. Several AEs present in the EV database are not reported in the registrational phase III studies. It is important to note that we have no information on the number of patients under treatment in the EV database.
CONCLUSIONS
The EV database highlights several AEs that are not reported in registrational phase III studies as well as different AEs profiles according to age. Clinicians should consider these data when treating patients with castration-resistant prostate cancer with ABI or ENZ.
中文翻译:

与阿比特龙和恩杂鲁胺治疗相关的不良事件:EudraVigilance数据库的分析和第三期注册研究的荟萃分析。
背景技术当在现实环境中使用时,来自临床试验的数据并不总是提供足够的信息来判断新疗法的影响。我们研究的目的是使用来自EudraVigilance(EV)数据库的真实数据分析与enzalutamide(ENZ)和abiraterone(ABI)相关的不良事件(AEs)。方法EV数据库是用于管理和分析有关已疑药物不良反应的信息的系统,该信息已被批准或正在欧洲经济区的临床试验中进行研究。我们记录了2013年1月至2019年1月每个类别和严重程度的ABI和ENZ的AE数量。此外,我们记录了每个年龄段的AE。进行了对注册三期研究(AFFIRM,PREVAIL,COU-AA)中报道的AE的荟萃分析。结果在EV数据库中识别出的个别病例数量对于ABI为13,562,对于ENZ为40,599。两种药物均将超过90%的报道不良事件定义为严重不良事件。与年轻患者相比,接受ABI或ENZ治疗的年龄较大的患者(> 85岁和65-85岁)患心脏病,传染病,代谢和呼吸系统疾病的风险增加(<65)。根据III期注册研究,ABI治疗患者中最常见的AE是肝胆疾病,而ENZ治疗患者中最常见的AE是精神病和血管疾病。登记数据库III期研究未报告EV数据库中存在的几种AE。重要的是要注意,EV数据库中没有关于正在接受治疗的患者人数的信息。结论EV数据库突出显示了在注册三期研究中未报告的几种AE,以及根据年龄不同的AE概况。当使用ABI或ENZ治疗去势抵抗性前列腺癌患者时,临床医生应考虑这些数据。
更新日期:2019-11-04
中文翻译:

与阿比特龙和恩杂鲁胺治疗相关的不良事件:EudraVigilance数据库的分析和第三期注册研究的荟萃分析。
背景技术当在现实环境中使用时,来自临床试验的数据并不总是提供足够的信息来判断新疗法的影响。我们研究的目的是使用来自EudraVigilance(EV)数据库的真实数据分析与enzalutamide(ENZ)和abiraterone(ABI)相关的不良事件(AEs)。方法EV数据库是用于管理和分析有关已疑药物不良反应的信息的系统,该信息已被批准或正在欧洲经济区的临床试验中进行研究。我们记录了2013年1月至2019年1月每个类别和严重程度的ABI和ENZ的AE数量。此外,我们记录了每个年龄段的AE。进行了对注册三期研究(AFFIRM,PREVAIL,COU-AA)中报道的AE的荟萃分析。结果在EV数据库中识别出的个别病例数量对于ABI为13,562,对于ENZ为40,599。两种药物均将超过90%的报道不良事件定义为严重不良事件。与年轻患者相比,接受ABI或ENZ治疗的年龄较大的患者(> 85岁和65-85岁)患心脏病,传染病,代谢和呼吸系统疾病的风险增加(<65)。根据III期注册研究,ABI治疗患者中最常见的AE是肝胆疾病,而ENZ治疗患者中最常见的AE是精神病和血管疾病。登记数据库III期研究未报告EV数据库中存在的几种AE。重要的是要注意,EV数据库中没有关于正在接受治疗的患者人数的信息。结论EV数据库突出显示了在注册三期研究中未报告的几种AE,以及根据年龄不同的AE概况。当使用ABI或ENZ治疗去势抵抗性前列腺癌患者时,临床医生应考虑这些数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号