Nature Catalysis ( IF 37.8 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41929-019-0375-7 Jia Zheng , Jira Jongcharoenkamol , Bram B. C. Peters , Jasper Guhl , Sudipta Ponra , Mårten S. G. Ahlquist , Pher G. Andersson
|
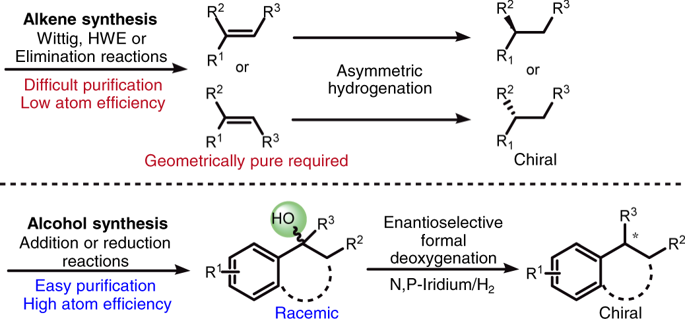
Asymmetric hydrogenation of alkenes is one of the most powerful tools for the preparation of optically active compounds. However, to achieve high enantioselectivity, the starting olefin in most cases needs to be isomerically pure in either the cis or the trans form. Generally, most olefination protocols provide olefins as isomeric mixtures that are difficult to separate, and in many cases also generate lots of waste. In contrast, the synthesis of racemic alcohols is straightforward and highly atom-efficient, with products that are easier to purify. Here, we describe a strategy that enables rapid access to chiral alkanes via enantioconvergent formal deoxygenation of racemic alcohols. Mechanistic studies indicate an Ir-mediated elimination of water and subsequent in situ hydrogenation. This approach allows rapid and efficient assembly of chiral intermediates and is exemplified in the total synthesis of antidepressant sertraline and σ2 receptor PB 28.
中文翻译:

铱催化的不对称氢化外消旋醇的对映体形式脱氧
烯烃的不对称氢化是制备旋光化合物最强大的工具之一。但是,为了实现高对映选择性,大多数情况下,起始烯烃在顺式或反式中都必须是异构体纯的形式。通常,大多数烯烃化方案以难以分离的异构混合物形式提供烯烃,并且在许多情况下还会产生大量废物。相反,外消旋醇的合成是直接且高度原子效率的,产物更易于纯化。在这里,我们描述了一种策略,该策略能够通过外消旋醇的对映体形式的正式脱氧来快速获得手性烷烃。机理研究表明Ir介导的水消除和随后的原位氢化。这种方法允许手性中间体的快速和有效的装配和在舍曲林的抗抑郁药的总合成和例举σ 2受体PB 28。


























 京公网安备 11010802027423号
京公网安备 11010802027423号