当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determination of physicochemical properties of aqueous mixture of sucrose with potassium chloride at 298.15 K: Water activity, osmotic coefficients, activity coefficients, solubility, excess Gibbs energies and transfer Gibbs energies
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.105962 Abdelfetah Mounir , Brahim Messnaoui , Abderrahim Dinane , Abderrahim Samaouali
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.jct.2019.105962 Abdelfetah Mounir , Brahim Messnaoui , Abderrahim Dinane , Abderrahim Samaouali
|
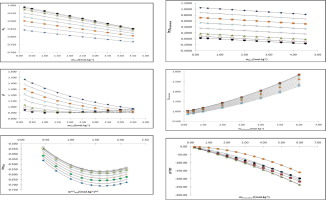
Abstract In this paper, we present the results of investigations of the KCl-D–sucrose-H2O ternary system using hygrometric method based on measurement of the relative humidity at the wide range of concentration to about saturation for both KCl and D–sucrose in different molalities of D–sucrose of (0.20, 0.50, 1.00, 2.00, 3.00, 4.00, 5.00 and 6.00) mol.kg−1 with the molality of KCl ranged from (0.20 to 4.50) mol.kg−1 at 298.15 K. The measured data are used to determine the water activities and osmotic coefficients. These studies are complemented using Pitzer-Simonson-Clegg model to understand different interactions involved between carbohydrates and KCl. Therefore, the water was considered as reference state in this study. Thus four mixture parameters are determined and used to predict the mean activity coefficients of KCl and D–sucrose. The solubility, excess Gibbs energy and transfer Gibbs energy are also calculated for this system.
中文翻译:

在 298.15 K 下测定蔗糖与氯化钾的含水混合物的物理化学性质:水活度、渗透系数、活度系数、溶解度、过量吉布斯能和转移吉布斯能
摘要 在本文中,我们介绍了使用湿度法研究 KCl-D-蔗糖-H2O 三元系统的结果,该方法基于测量不同浓度的 KCl 和 D-蔗糖在宽浓度范围内的相对湿度至饱和状态。 D-蔗糖的摩尔浓度为 (0.20, 0.50, 1.00, 2.00, 3.00, 4.00, 5.00 和 6.00) mol.kg-1,KCl 的摩尔浓度范围为 (0.20 到 4.50) mol.kg-1 at The K2.9测量数据用于确定水分活度和渗透系数。这些研究使用 Pitzer-Simonson-Clegg 模型进行补充,以了解碳水化合物和 KCl 之间涉及的不同相互作用。因此,在本研究中将水视为参考状态。因此,确定了四个混合物参数并用于预测 KCl 和 D-蔗糖的平均活性系数。
更新日期:2020-01-01
中文翻译:

在 298.15 K 下测定蔗糖与氯化钾的含水混合物的物理化学性质:水活度、渗透系数、活度系数、溶解度、过量吉布斯能和转移吉布斯能
摘要 在本文中,我们介绍了使用湿度法研究 KCl-D-蔗糖-H2O 三元系统的结果,该方法基于测量不同浓度的 KCl 和 D-蔗糖在宽浓度范围内的相对湿度至饱和状态。 D-蔗糖的摩尔浓度为 (0.20, 0.50, 1.00, 2.00, 3.00, 4.00, 5.00 和 6.00) mol.kg-1,KCl 的摩尔浓度范围为 (0.20 到 4.50) mol.kg-1 at The K2.9测量数据用于确定水分活度和渗透系数。这些研究使用 Pitzer-Simonson-Clegg 模型进行补充,以了解碳水化合物和 KCl 之间涉及的不同相互作用。因此,在本研究中将水视为参考状态。因此,确定了四个混合物参数并用于预测 KCl 和 D-蔗糖的平均活性系数。


























 京公网安备 11010802027423号
京公网安备 11010802027423号