当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water in protein hydration and ligand recognition.
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-08-27 , DOI: 10.1002/jmr.2810 Manuela Maurer 1 , Chris Oostenbrink 1
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-08-27 , DOI: 10.1002/jmr.2810 Manuela Maurer 1 , Chris Oostenbrink 1
Affiliation
|
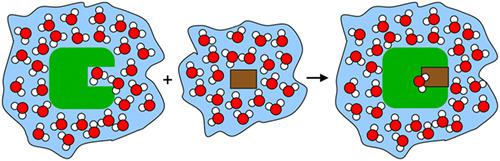
This review describes selected basics of water in biomolecular recognition. We focus on a qualitative understanding of the most important physical aspects, how these change in magnitude between bulk water and protein environment, and how the roles that water plays for proteins arise from them. These roles include mechanical support, thermal coupling, dielectric screening, mass and charge transport, and the competition with a ligand for the occupation of a binding site. The presence or absence of water has ramifications that range from the thermodynamic binding signature of a single ligand up to cellular survival. The large inhomogeneity in water density, polarity and mobility around a solute is hard to assess in experiment. This is a source of many difficulties in the solvation of protein models and computational studies that attempt to elucidate or predict ligand recognition. The influence of water in a protein binding site on the experimental enthalpic and entropic signature of ligand binding is still a point of much debate. The strong water-water interaction in enthalpic terms is counteracted by a water molecule's high mobility in entropic terms. The complete arrest of a water molecule's mobility sets a limit on the entropic contribution of a water displacement process, while the solvent environment sets limits on ligand reactivity.
中文翻译:

蛋白质水合和配体识别中的水。
这篇综述描述了生物分子识别中水的选定基础知识。我们专注于对最重要的物理方面的定性理解,这些方面如何在散装水和蛋白质环境之间发生变化,以及水对蛋白质所起的作用是如何产生的。这些作用包括机械支持、热耦合、介电屏蔽、质量和电荷传输,以及与配体竞争结合位点。水的存在与否具有从单个配体的热力学结合特征到细胞存活的范围。在实验中很难评估溶质周围的水密度、极性和流动性的巨大不均匀性。这是试图阐明或预测配体识别的蛋白质模型和计算研究中的许多困难的根源。蛋白质结合位点中的水对配体结合的实验焓和熵特征的影响仍然是一个备受争议的问题。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。
更新日期:2019-08-27
中文翻译:

蛋白质水合和配体识别中的水。
这篇综述描述了生物分子识别中水的选定基础知识。我们专注于对最重要的物理方面的定性理解,这些方面如何在散装水和蛋白质环境之间发生变化,以及水对蛋白质所起的作用是如何产生的。这些作用包括机械支持、热耦合、介电屏蔽、质量和电荷传输,以及与配体竞争结合位点。水的存在与否具有从单个配体的热力学结合特征到细胞存活的范围。在实验中很难评估溶质周围的水密度、极性和流动性的巨大不均匀性。这是试图阐明或预测配体识别的蛋白质模型和计算研究中的许多困难的根源。蛋白质结合位点中的水对配体结合的实验焓和熵特征的影响仍然是一个备受争议的问题。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。焓项中的强水-水相互作用被熵项中水分子的高迁移率所抵消。水分子流动性的完全停滞限制了水置换过程的熵贡献,而溶剂环境限制了配体反应性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号