当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanoclay montmorillonite as an adsorbent for CO2 capture: Experimental and modeling
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-08-06 , DOI: 10.1002/jccs.201900150 Mojtaba Khajeh 1 , Ahad Ghaemi 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-08-06 , DOI: 10.1002/jccs.201900150 Mojtaba Khajeh 1 , Ahad Ghaemi 1
Affiliation
|
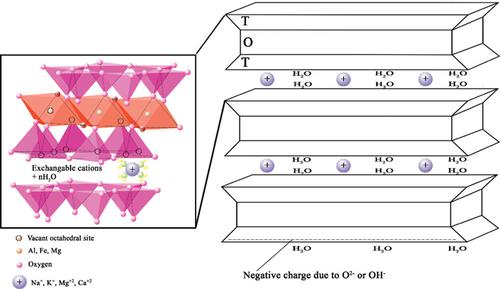
Today, the tendency to use mineral adsorbents has increased due to its low cost for removal of the flue gas of fossil‐fuel power plants. In this research, the optimization of the CO2 adsorption process using the sodium‐montmorillonite adsorbent was investigated. The response surface methodology (RSM), combined with central composite design, was used to assess the effects of process variables and their interaction on the response (CO2 adsorption capacity) to achieve the optimal conditions. According to the analysis of variance, temperature and pressure parameters are the variables that affect the CO2 adsorption capacity. Moreover, one semi‐empirical correlation was established to calculate the optimum operating conditions of the CO2 adsorption process. The optimal variables of the process obtained from numerical optimization are 25°C and 9 bar for temperature and pressure, respectively. Based on the optimal conditions, the adsorption capacity of 100.67 mg/g was achieved. Furthermore, additional experiments were performed to examine the isotherm, kinetic, and thermodynamic models of absorption. Kinetic studies showed that adsorption was in accordance with the second‐order reaction. Equilibrium data were analyzed using Langmuir, Freundlich, Hill, and D‐R isotherm models at different temperatures. The D‐R isotherm provides the best description for the adsorption experimental data.
中文翻译:

纳米粘土蒙脱石作为二氧化碳捕集的吸附剂:实验和建模
如今,由于矿物吸附剂去除化石燃料发电厂的烟道气的成本低廉,因此使用矿物吸附剂的趋势有所增加。在这项研究中,研究了使用钠-蒙脱土吸附剂优化CO 2吸附过程的方法。响应面方法(RSM)与中心复合设计相结合,用于评估过程变量及其相互作用对响应(CO 2吸附容量)的影响,以达到最佳条件。根据方差分析,温度和压力参数是影响CO 2吸附容量的变量。此外,建立了一种半经验相关性以计算CO 2的最佳运行条件吸附过程。通过数值优化获得的最佳工艺变量的温度和压力分别为25°C和9 bar。在最佳条件下,吸附量达到了100.67 mg / g。此外,还进行了其他实验以检查吸收的等温线,动力学和热力学模型。动力学研究表明,吸附符合二级反应。使用Langmuir,Freundlich,Hill和D‐R等温线模型在不同温度下分析平衡数据。D‐R等温线为吸附实验数据提供了最好的描述。
更新日期:2020-02-14
中文翻译:

纳米粘土蒙脱石作为二氧化碳捕集的吸附剂:实验和建模
如今,由于矿物吸附剂去除化石燃料发电厂的烟道气的成本低廉,因此使用矿物吸附剂的趋势有所增加。在这项研究中,研究了使用钠-蒙脱土吸附剂优化CO 2吸附过程的方法。响应面方法(RSM)与中心复合设计相结合,用于评估过程变量及其相互作用对响应(CO 2吸附容量)的影响,以达到最佳条件。根据方差分析,温度和压力参数是影响CO 2吸附容量的变量。此外,建立了一种半经验相关性以计算CO 2的最佳运行条件吸附过程。通过数值优化获得的最佳工艺变量的温度和压力分别为25°C和9 bar。在最佳条件下,吸附量达到了100.67 mg / g。此外,还进行了其他实验以检查吸收的等温线,动力学和热力学模型。动力学研究表明,吸附符合二级反应。使用Langmuir,Freundlich,Hill和D‐R等温线模型在不同温度下分析平衡数据。D‐R等温线为吸附实验数据提供了最好的描述。


























 京公网安备 11010802027423号
京公网安备 11010802027423号