当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent effects on the geometry, electronic structure, and bonding style of Zn(N5)2: A theoretical study
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-07-04 , DOI: 10.1002/jccs.201900205 Zhiyuan Ding 1 , Pin Gao 2 , Ming Lu 1 , Guixiang Wang 1 , Xuedong Gong 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-07-04 , DOI: 10.1002/jccs.201900205 Zhiyuan Ding 1 , Pin Gao 2 , Ming Lu 1 , Guixiang Wang 1 , Xuedong Gong 1
Affiliation
|
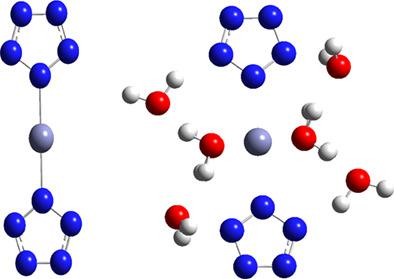
Nitrogen‐rich compounds involving the cyclo‐pentazole anion (cyclo‐N5−) have attracted extensive attention due to higher energy release and environmental friendliness than traditional high energy density materials (HEDMs). However, the synthesis of stable HEDMs with cyclo‐N5− is still a challenge. In this study, the effect of nine solvents on the geometrical and electronic structures and solvation energies of Zn(N5)2, one of the recently synthesized nitrogen‐rich compounds, was studied using the density functional theory and the polarized continuum model. The results indicated an increase in the stability of Zn(N5)2 in the solution phase compared to the vacuum phase, and the stability of Zn(N5)2 increases with increasing dielectric constants. The energy gap of frontier molecular orbitals and the absolute value of total energy in water are the largest, revealing that Zn(N5)2 is more stable in water than in other solvents. To understand the stabilization mechanism of Zn(N5)2 by water, further studies were performed with the natural bond orbital (NBO) analysis and the quantum theory of atoms in molecules (QTAIM) analysis using the explicit solvent model. The charge transfer and the hydrogen bonds are observed between Zn(N5)2 and water, which are beneficial to improvement of the stability of Zn(N5)2. This may indicate the solvents that have strong interactions with the cyclo‐N5− candidate may improve the possibility of success of synthesis.
中文翻译:

溶剂对Zn(N5)2的几何构型,电子结构和键合样式的影响:理论研究
涉及环五唑阴离子(环-N富氮化合物5 - )已经吸引了广泛的关注是由于较高的能量释放和环境友好性比传统的高能量密度的材料(HEDMs)。然而,随着环-N稳定HEDMs的合成5 -仍然是一个挑战。在这项研究中,使用密度泛函理论和极化连续谱模型研究了九种溶剂对Zn(N 5)2(一种最近合成的富氮化合物)的几何和电子结构以及溶剂化能的影响。结果表明增加了Zn(N 5)2的稳定性与真空相相比,在溶液相中Zn(N 5)2的稳定性随介电常数的增加而增加。前沿分子轨道的能隙和水中的总能量绝对值最大,这表明Zn(N 5)2在水中比在其他溶剂中更稳定。为了解水对Zn(N 5)2的稳定机理,使用显式溶剂模型对自然键轨道(NBO)分析和分子中原子的量子理论(QTAIM)分析进行了进一步的研究。在Zn(N 5)2之间观察到电荷转移和氢键和水,有利于提高Zn(N 5)2的稳定性。这可能表明,具有与环-N强相互作用的溶剂5 -候选者可提高合成的成功的可能性。
更新日期:2020-02-14
中文翻译:

溶剂对Zn(N5)2的几何构型,电子结构和键合样式的影响:理论研究
涉及环五唑阴离子(环-N富氮化合物5 - )已经吸引了广泛的关注是由于较高的能量释放和环境友好性比传统的高能量密度的材料(HEDMs)。然而,随着环-N稳定HEDMs的合成5 -仍然是一个挑战。在这项研究中,使用密度泛函理论和极化连续谱模型研究了九种溶剂对Zn(N 5)2(一种最近合成的富氮化合物)的几何和电子结构以及溶剂化能的影响。结果表明增加了Zn(N 5)2的稳定性与真空相相比,在溶液相中Zn(N 5)2的稳定性随介电常数的增加而增加。前沿分子轨道的能隙和水中的总能量绝对值最大,这表明Zn(N 5)2在水中比在其他溶剂中更稳定。为了解水对Zn(N 5)2的稳定机理,使用显式溶剂模型对自然键轨道(NBO)分析和分子中原子的量子理论(QTAIM)分析进行了进一步的研究。在Zn(N 5)2之间观察到电荷转移和氢键和水,有利于提高Zn(N 5)2的稳定性。这可能表明,具有与环-N强相互作用的溶剂5 -候选者可提高合成的成功的可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号