当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and evaluation of pyrazole‐incorporated monocarbonyl curcumin analogues as antiproliferative and antioxidant agents
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-03-25 , DOI: 10.1002/jccs.201800405 Amol A. Nagargoje 1 , Satish V. Akolkar 1 , Madiha M. Siddiqui 1 , Aditi V. Bagade 2 , Kisan M. Kodam 2 , Jaiprakash N. Sangshetti 3 , Manoj G. Damale 4 , Bapurao B. Shingate 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2019-03-25 , DOI: 10.1002/jccs.201800405 Amol A. Nagargoje 1 , Satish V. Akolkar 1 , Madiha M. Siddiqui 1 , Aditi V. Bagade 2 , Kisan M. Kodam 2 , Jaiprakash N. Sangshetti 3 , Manoj G. Damale 4 , Bapurao B. Shingate 1
Affiliation
|
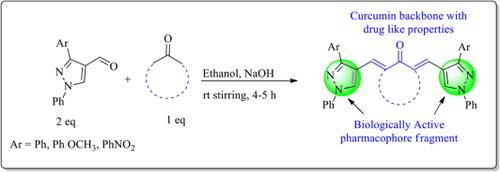
A series of pyrazole‐incorporated monocarbonyl analogues of curcumin were synthesized via Clasien–Schimidt‐type condensation and subsequently screened for in vitro antiproliferative and antioxidant activity. The analogues 4c, 5d, 5e, 5g, 6e, and 6f showed potential activity against the MDA‐MB‐231 cell line. The synthesized analogues were also screened for their antioxidant activity. Compounds 5a, 5e, 6d, and 6f exhibit comparable radical scavenging activity with respect to the standard drug ascorbic acid. Furthermore, a molecular docking study has been conducted for 5d and 5g and suggests that these compounds have a potential to become lead molecules in drug discovery and process.
中文翻译:

吡唑并入的单羰基姜黄素类似物的合成和评估作为抗增殖剂和抗氧化剂
通过Clasien–Schimidt型缩合反应合成了一系列并入吡唑的姜黄素单羰基类似物,随后筛选了其体外抗增殖和抗氧化活性。类似物4c,5d,5e,5g,6e和6f对MDA-MB-231细胞系显示出潜在的活性。还筛选了合成的类似物的抗氧化活性。相对于标准药物抗坏血酸,化合物5a,5e,6d和6f表现出相当的自由基清除活性。此外,进行了5d和5g的分子对接研究 并暗示这些化合物有可能成为药物发现和加工过程中的先导分子。
更新日期:2019-12-11
中文翻译:

吡唑并入的单羰基姜黄素类似物的合成和评估作为抗增殖剂和抗氧化剂
通过Clasien–Schimidt型缩合反应合成了一系列并入吡唑的姜黄素单羰基类似物,随后筛选了其体外抗增殖和抗氧化活性。类似物4c,5d,5e,5g,6e和6f对MDA-MB-231细胞系显示出潜在的活性。还筛选了合成的类似物的抗氧化活性。相对于标准药物抗坏血酸,化合物5a,5e,6d和6f表现出相当的自由基清除活性。此外,进行了5d和5g的分子对接研究 并暗示这些化合物有可能成为药物发现和加工过程中的先导分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号