Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preprotein signature for full susceptibility to the co-translational translocation inhibitor cyclotriazadisulfonamide.
Traffic ( IF 4.5 ) Pub Date : 2019-11-01 , DOI: 10.1111/tra.12713 Victor Van Puyenbroeck 1 , Eva Pauwels 1 , Becky Provinciael 1 , Thomas W Bell 2 , Dominique Schols 1 , Kai-Uwe Kalies 3 , Enno Hartmann 3 , Kurt Vermeire 1
Traffic ( IF 4.5 ) Pub Date : 2019-11-01 , DOI: 10.1111/tra.12713 Victor Van Puyenbroeck 1 , Eva Pauwels 1 , Becky Provinciael 1 , Thomas W Bell 2 , Dominique Schols 1 , Kai-Uwe Kalies 3 , Enno Hartmann 3 , Kurt Vermeire 1
Affiliation
|
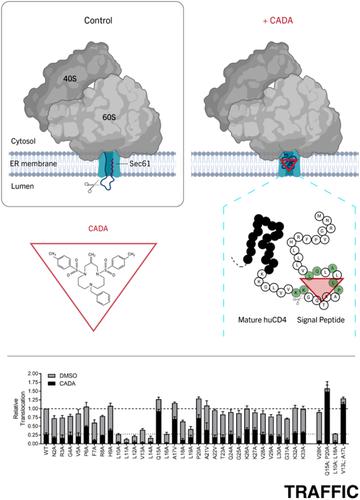
Cyclotriazadisulfonamide (CADA) inhibits the co-translational translocation of human CD4 (huCD4) into the endoplasmic reticulum lumen in a signal peptide (SP)-dependent way. We propose that CADA binds the nascent huCD4 SP in a folded conformation within the translocon resembling a normally transitory state during translocation. Here, we used alanine scanning on the huCD4 SP to identify the signature for full susceptibility to CADA. In accordance with our previous work, we demonstrate that residues in the vicinity of the hydrophobic h-region are critical for sensitivity to CADA. In particular, exchanging Gln-15, Val-17 or Pro-20 in the huCD4 SP for Ala resulted in a resistant phenotype. Together with positively charged residues at the N-terminal portion of the mature protein, these residues mediate full susceptibility to the co-translational translocation inhibitory activity of CADA towards huCD4. In addition, sensitivity to CADA is inversely related to hydrophobicity in the huCD4 SP. In vitro translocation experiments confirmed that the general hydrophobicity of the h-domain and positive charges in the mature protein are key elements that affect both the translocation efficiency of huCD4 and the sensitivity towards CADA. Besides these two general SP parameters that determine the functionality of the signal sequence, unique amino acid pairs (L14/Q15 and L19/P20) in the SP hydrophobic core add specificity to the sensitivity signature for a co-translational translocation inhibitor.
中文翻译:

预蛋白签名对共翻译易位抑制剂环三氮杂二磺酰胺具有完全敏感性。
环三氮杂二磺酰胺(CADA)以信号肽(SP)依赖性方式抑制人CD4(huCD4)共同翻译进入内质网腔。我们建议CADA结合新生的huCD4 SP的折叠构象内的转座子,类似于易位期间的正常过渡状态。在这里,我们在huCD4 SP上使用了丙氨酸扫描,以识别出对CADA完全易感的特征。根据我们以前的工作,我们证明了疏水性h区附近的残基对于CADA的敏感性至关重要。特别是,在huCD4 SP中将Gln-15,Val-17或Pro-20交换为Ala会产生抗性表型。加上成熟蛋白质N端带正电荷的残基,这些残基介导了对CADA对huCD4的共翻译易位抑制活性的完全敏感性。另外,对CADA的敏感性与huCD4 SP中的疏水性成反比。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)还增加了共翻译易位抑制剂敏感性的特异性。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)还增加了共翻译易位抑制剂敏感性的特异性。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)为共翻译易位抑制剂的敏感性特征增加了特异性。
更新日期:2020-01-21
中文翻译:

预蛋白签名对共翻译易位抑制剂环三氮杂二磺酰胺具有完全敏感性。
环三氮杂二磺酰胺(CADA)以信号肽(SP)依赖性方式抑制人CD4(huCD4)共同翻译进入内质网腔。我们建议CADA结合新生的huCD4 SP的折叠构象内的转座子,类似于易位期间的正常过渡状态。在这里,我们在huCD4 SP上使用了丙氨酸扫描,以识别出对CADA完全易感的特征。根据我们以前的工作,我们证明了疏水性h区附近的残基对于CADA的敏感性至关重要。特别是,在huCD4 SP中将Gln-15,Val-17或Pro-20交换为Ala会产生抗性表型。加上成熟蛋白质N端带正电荷的残基,这些残基介导了对CADA对huCD4的共翻译易位抑制活性的完全敏感性。另外,对CADA的敏感性与huCD4 SP中的疏水性成反比。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)还增加了共翻译易位抑制剂敏感性的特异性。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)还增加了共翻译易位抑制剂敏感性的特异性。体外易位实验证实,成熟蛋白中h结构域的一般疏水性和正电荷是影响huCD4易位效率和对CADA敏感性的关键因素。除了确定信号序列功能的这两个通用SP参数外,SP疏水核心中的独特氨基酸对(L14 / Q15和L19 / P20)为共翻译易位抑制剂的敏感性特征增加了特异性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号