JAMA Dermatology ( IF 10.9 ) Pub Date : 2019-10-30 , DOI: 10.1001/jamadermatol.2019.3270 John S Barbieri 1 , Ilona J Frieden 2 , Arielle R Nagler 3
|
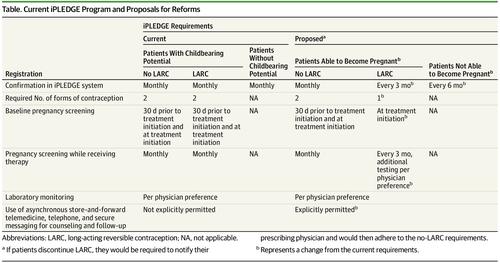
Isotretinoin is a highly effective treatment for acne and is unique because it can reliably lead to disease remission. It is also teratogenic, and for this reason access to the medication has been regulated by a series of increasingly stringent pregnancy prevention programs during the past 3 decades. The current program, implemented in 2006, is called iPLEDGE.1 It is an example of a Risk Evaluation and Mitigation Strategy program, which are drug safety programs that the US Food and Drug Administration (FDA) requires for medications with serious safety concerns. Required registration of isotretinoin users in iPLEDGE is distinct from other teratogenic medications commonly used in the field of dermatology, such as acitretin and methotrexate, which do not have similar FDA-instituted Risk Evaluation and Mitigation Strategy pregnancy prevention programs. Fetal exposures to isotretinoin, however, have continued to occur since iPLEDGE’s introduction,2 with no difference in frequency of fetal exposures before and after the program’s rollout.1 Not only is iPLEDGE ineffective, but its requirements have caused unintended harm to patients and placed a major administrative burden on both prescribers and patients. In this Viewpoint, we review the harm caused by iPLEDGE and propose reforms to optimize its contraception requirements and ease its administrative burden.
中文翻译:

异维A酸,患者安全性和以患者为中心的护理时间来改革iPLEDGE。
异维A酸是治疗痤疮的高效方法,并且独特,因为它可以可靠地导致疾病缓解。它也具有致畸性,因此,在过去的30年中,一系列越来越严格的预防怀孕计划对药物的使用进行了管制。当前计划于2006年实施,称为iPLEDGE。1个它是“风险评估和缓解策略”计划的一个示例,该计划是美国食品和药物管理局(FDA)对于具有严重安全隐患的药物所要求的药物安全计划。异维A酸使用者在iPLEDGE中所需的注册不同于皮肤病学领域常用的其他致畸药物,例如阿维A和氨甲蝶呤,它们没有类似的FDA制定的风险评估和缓解策略怀孕预防计划。但是,自从iPLEDGE推出以来,异维A酸的胎儿接触一直持续发生2,在该计划推出之前和之后,胎儿接触频率的差异均没有差异。1个iPLEDGE不仅无效,而且其要求已给患者带来意想不到的伤害,并给开处方者和患者带来重大的行政负担。在这种观点下,我们回顾了iPLEDGE所造成的危害,并提出了改革措施,以优化其避孕要求并减轻其行政负担。



























 京公网安备 11010802027423号
京公网安备 11010802027423号