JAMA Oncology ( IF 28.4 ) Pub Date : 2020-01-01 , DOI: 10.1001/jamaoncol.2019.3718 Andrea Cercek 1 , Thomas Boerner 2 , Benjamin R Tan 3 , Joanne F Chou 4 , Mithat Gönen 4 , Taryn M Boucher 1 , Haley F Hauser 1 , Richard K G Do 5 , Maeve A Lowery 1 , James J Harding 1 , Anna M Varghese 1 , Diane Reidy-Lagunes 1 , Leonard Saltz 1 , Nikolaus Schultz 6 , T Peter Kingham 2 , Michael I D'Angelica 2 , Ronald P DeMatteo 7 , Jeffrey A Drebin 2 , Peter J Allen 8 , Vinod P Balachandran 2 , Kian-Huat Lim 3 , Francisco Sanchez-Vega 9 , Neeta Vachharajani 9 , Maria B Majella Doyle 9 , Ryan C Fields 9 , William G Hawkins 9 , Steven M Strasberg 9 , William C Chapman 9 , Luis A Diaz 1 , Nancy E Kemeny 1 , William R Jarnagin 2
|
Importance Unresectable intrahepatic cholangiocarcinoma (IHC) carries a poor prognosis, with a median overall survival (OS) of 11 months. Hepatic arterial infusion (HAI) of high-dose chemotherapy may have potential benefit in these patients.
Objective To evaluate clinical outcomes when HAI chemotherapy is combined with systemic chemotherapy in patients with unresectable IHC.
Design, Setting, and Participants A single-institution, phase 2 clinical trial including 38 patients was conducted with HAI floxuridine plus systemic gemcitabine and oxaliplatin in patients with unresectable IHC at Memorial Sloan Kettering Cancer Center between May 20, 2013, and June 27, 2019. A confirmatory phase 1/2 study using the same therapy was conducted during the same time period at Washington University in St Louis. Patients with histologically confirmed, unresectable IHC were eligible. Resectable metastatic disease to regional lymph nodes and prior systemic therapy were permitted. Patients with distant metastatic disease were excluded.
Interventions Hepatic arterial infusion of floxuridine and systemic administration of gemcitabine and oxaliplatin.
Main Outcomes and Measures The primary outcome was progression-free survival (PFS) of 80% at 6 months.
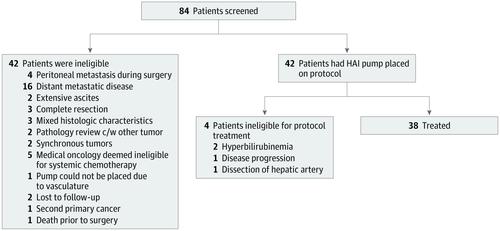
Results For the phase 2 clinical trial at Memorial Sloan Kettering Cancer Center, 42 patients with unresectable IHC were included and, of these, 38 patients were treated (13 [34%] men; median [range] age at diagnosis, 64 [39-81] years). The median follow-up was 30.5 months. Twenty-two patients (58%) achieved a partial radiographic response, and 32 patients (84%) achieved disease control at 6 months. Four patients had sufficient response to undergo resection, and 1 patient had a complete pathologic response. The median PFS was 11.8 months (1-sided 90% CI, 11.1) with a 6-month PFS rate of 84.1% (90% CI, 74.8%-infinity), thereby meeting the primary end point (6-month PFS rate, 80%). The median OS was 25.0 months (95% CI, 20.6-not reached), and the 1-year OS rate was 89.5% (95% CI, 80.2%-99.8%). Patients with resectable regional lymph nodes (18 [47%]) showed no difference in OS compared with patients with node-negative disease (24-month OS: lymph node negative: 60%; 95% CI, 40%-91% vs lymph node positive: 50%; 95% CI, 30%-83%; P = .66). Four patients (11%) had grade 4 toxic effects requiring removal from the study (1 portal hypertension, 2 gastroduodenal artery aneurysms, 1 infection in the pump pocket). Subgroup analysis showed significant improvement in survival in patients with IDH1/2 mutated tumors (2-year OS, 90%; 95% CI, 73%-99%) vs wild-type (2-year OS, 33%; 95% CI, 18%-63%) (P = .01). In the Washington University in St Louis confirmatory cohort, 9 patients (90%) achieved disease control at 6 months; the most common grade 3 toxic effect was elevated results of liver function tests, and median PFS was 12.8 months (1-sided 90% CI, 6.4).
Conclusions and Relevance Hepatic arterial infusion plus systemic chemotherapy appears to be highly active and tolerable in patients with unresectable IHC; further evaluation is warranted.
中文翻译:

在不可切除的肝内胆管癌患者中评估氟尿苷与全身性吉西他滨和奥沙利铂的肝动脉输注:2 期临床试验。
重要性 不可切除的肝内胆管癌 (IHC) 预后不良,中位总生存期 (OS) 为 11 个月。大剂量化疗的肝动脉灌注 (HAI) 可能对这些患者有潜在的益处。
目的 评价HAI化疗联合全身化疗对不可切除IHC患者的临床疗效。
设计、设置和参与者 2013 年 5 月 20 日至 2019 年 6 月 27 日期间,在纪念斯隆凯特琳癌症中心,对无法切除的 IHC 患者使用 HAI 氟尿苷联合全身吉西他滨和奥沙利铂进行了一项包括 38 名患者的单机构 2 期临床试验. 同一时期在圣路易斯华盛顿大学进行了一项使用相同疗法的确认性 1/2 期研究。具有组织学证实的、不可切除的 IHC 的患者符合条件。允许可切除的区域淋巴结转移性疾病和先前的全身治疗。排除有远处转移性疾病的患者。
干预 氟尿苷的肝动脉输注和吉西他滨和奥沙利铂的全身给药。
主要结果和测量 主要结果是 6 个月时 80% 的无进展生存期 (PFS)。
结果 在纪念斯隆凯特琳癌症中心的 2 期临床试验中,纳入了 42 名无法切除的 IHC 患者,其中 38 名患者接受了治疗(13 [34%] 男性;诊断时的中位 [范围] 年龄,64 [39-81 ] 年)。中位随访时间为 30.5 个月。22 名患者 (58%) 在 6 个月时实现了部分放射学缓解,32 名患者 (84%) 实现了疾病控制。4名患者有足够的反应进行切除,1名患者有完全的病理反应。中位 PFS 为 11.8 个月(单侧 90% CI,11.1),6 个月 PFS 率为 84.1%(90% CI,74.8%-无穷大),从而达到主要终点(6 个月 PFS 率, 80%)。中位 OS 为 25.0 个月(95% CI,20.6-未达到),1 年 OS 率为 89.5%(95% CI,80.2%-99.8%)。P = .66)。4 名患者 (11%) 有 4 级毒性反应需要从研究中移除(1 名门静脉高压症、2 名胃十二指肠动脉瘤、1 名泵袋感染)。亚组分析显示IDH1/2突变肿瘤患者(2 年 OS,90%;95% CI,73%-99%)与野生型(2 年 OS,33%;95% CI)相比,生存率显着提高, 18%-63%) ( P = .01)。在圣路易斯华盛顿大学的确认队列中,9 名患者(90%)在 6 个月时实现了疾病控制;最常见的 3 级毒性作用是肝功能检查结果升高,中位 PFS 为 12.8 个月(单侧 90% CI,6.4)。
结论和相关性 肝动脉灌注加全身化疗似乎对不可切除的 IHC 患者具有高度活性和耐受性;需要进一步评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号