当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial.
JAMA Oncology ( IF 28.4 ) Pub Date : 2019-10-31 , DOI: 10.1001/jamaoncol.2019.3702 Julien Edeline 1, 2 , Yann Touchefeu 3 , Boris Guiu 4 , Olivier Farge 5 , David Tougeron 6 , Isabelle Baumgaertner 7 , Ahmet Ayav 8 , Boris Campillo-Gimenez 1, 9 , Luc Beuzit 10 , Marc Pracht 1 , Astrid Lièvre 11, 12 , Samuel Le Sourd 1 , Karim Boudjema 13 , Yan Rolland 14 , Eveline Boucher 1 , Etienne Garin 15
JAMA Oncology ( IF 28.4 ) Pub Date : 2019-10-31 , DOI: 10.1001/jamaoncol.2019.3702 Julien Edeline 1, 2 , Yann Touchefeu 3 , Boris Guiu 4 , Olivier Farge 5 , David Tougeron 6 , Isabelle Baumgaertner 7 , Ahmet Ayav 8 , Boris Campillo-Gimenez 1, 9 , Luc Beuzit 10 , Marc Pracht 1 , Astrid Lièvre 11, 12 , Samuel Le Sourd 1 , Karim Boudjema 13 , Yan Rolland 14 , Eveline Boucher 1 , Etienne Garin 15
Affiliation
|
Importance
Patients with unresectable intrahepatic cholangiocarcinoma (ICC) have a poor prognosis. Selective internal radiotherapy (SIRT) is a promising treatment option for hepatic tumors, but no prospective studies of combination SIRT with chemotherapy have been published to our knowledge.
Objective
To determine the response rate after SIRT combined with chemotherapy in patients with unresectable ICC.
Design, Setting, and Participants
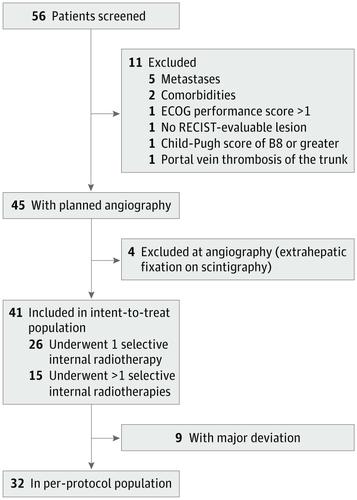
This phase 2 clinical trial, the Yttrium-90 Microspheres in Cholangiocarcinoma (MISPHEC) trial, included patients with unresectable ICC who have never received chemotherapy or intra-arterial therapy and were treated at 7 centers which had experience with SIRT between November 12, 2013, and June 21, 2016. Statistical analysis was performed from March 31, 2017, to June 17, 2019.
Interventions
Concomitant first-line chemotherapy with cisplatin, 25 mg/m2, and gemcitabine, 1000 mg/m2 (gemcitabine reduced to 300 mg/m2 for the cycles just before and after SIRT), on days 1 and 8 of a 21-day cycle for 8 cycles. Selective internal radiotherapy was administered during cycle 1 (1 hemiliver disease) or cycles 1 and 3 (disease involving both hemilivers) using glass Y90 microspheres.
Main Outcomes and Measures
Response rate at 3 months according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Secondary end points were toxic effects, progression-free survival, overall survival, disease control rate, and response rate according to Choi criteria.
Results
Of 41 patients included in the study, 26 (63%) were male, with a mean (SD) age of 64.0 (10.7) years. Response rate according to RECIST was 39% (90% CI, 26%-53%) at 3 months according to local review and was confirmed at 41% as best response by central review; disease control rate was 98%. According to Choi criteria, the response rate was 93%. After a median follow-up of 36 months (95% CI, 26-52 months), median progression-free survival was 14 months (95% CI, 8-17 months), with progression-free survival rates of 55% at 12 months and 30% at 24 months. Median overall survival was 22 months (95% CI, 14-52 months), with overall survival rates of 75% at 12 months and 45% at 24 months. Of 41 patients, 29 (71%) had grades 3 to 4 toxic effects; 9 patients (22%) could be downstaged to surgical intervention, with 8 (20%) achieving R0 (microscopic-free margins) surgical resection. After a median of 46 months (95% CI, 31 months to not reached) after surgery, median relapse-free survival was not reached among patients who underwent resection.
Conclusions and Relevance
Combination chemotherapy and SIRT had antitumor activity as first-line treatment of unresectable ICC, and a significant proportion of patients were downstaged to surgical intervention. A phase 3 trial is ongoing.
中文翻译:

放射栓塞加化疗一线治疗局部晚期肝内胆管癌:2 期临床试验。
重要性 不可切除的肝内胆管癌 (ICC) 患者预后较差。选择性体内放疗 (SIRT) 是一种很有前途的肝肿瘤治疗选择,但据我们所知,尚无 SIRT 与化疗联合的前瞻性研究发表。目的探讨SIRT联合化疗对不可切除ICC患者的反应率。设计、设置和参与者 该 2 期临床试验,即 Yttrium-90 微球治疗胆管癌 (MISPHEC) 试验,纳入了从未接受过化疗或动脉内治疗且在 7 个有 SIRT 经验的中心接受治疗的不可切除 ICC 患者2013 年 11 月 12 日至 2016 年 6 月 21 日。统计分析时间为 2017 年 3 月 31 日至 2019 年 6 月 17 日。在 21 天周期的第 1 天和第 8 天,同时使用顺铂 25 mg/m2 和吉西他滨 1000 mg/m2 的一线化疗(在 SIRT 之前和之后的周期中,吉西他滨降低至 300 mg/m2) 8个周期。在第 1 周期(1 个半肝疾病)或第 1 和第 3 周期(涉及两个半肝的疾病)期间使用玻璃 Y90 微球进行选择性内部放射治疗。主要结果和措施 根据实体瘤反应评估标准 (RECIST) 1.1 在 3 个月时的反应率。根据 Choi 标准,次要终点是毒性作用、无进展生存期、总生存期、疾病控制率和缓解率。结果 纳入研究的 41 名患者中,26 名 (63%) 为男性,平均 (SD) 年龄为 64.0 (10.7) 岁。根据 RECIST 的反应率为 39%(90% CI,26%-53%)在 3 个月时根据当地审查确定,41% 被中央审查确认为最佳响应;疾病控制率为98%。根据Choi标准,响应率为93%。中位随访 36 个月(95% CI,26-52 个月)后,中位无进展生存期为 14 个月(95% CI,8-17 个月),12 岁时无进展生存率为 55%个月和 24 个月时为 30%。中位总生存期为 22 个月(95% CI,14-52 个月),12 个月和 24 个月的总生存率为 75% 和 45%。在 41 名患者中,29 名(71%)有 3 至 4 级毒性反应;9 名患者 (22%) 可降级为手术干预,其中 8 名 (20%) 达到 R0(无显微镜下切缘)手术切除。术后中位 46 个月(95% CI,31 个月至未达到)后,接受切除术的患者未达到中位无复发生存期。结论和相关性 联合化疗和 SIRT 作为不可切除 ICC 的一线治疗具有抗肿瘤活性,并且相当一部分患者降期至手术干预。3期试验正在进行中。
更新日期:2020-01-09
中文翻译:

放射栓塞加化疗一线治疗局部晚期肝内胆管癌:2 期临床试验。
重要性 不可切除的肝内胆管癌 (ICC) 患者预后较差。选择性体内放疗 (SIRT) 是一种很有前途的肝肿瘤治疗选择,但据我们所知,尚无 SIRT 与化疗联合的前瞻性研究发表。目的探讨SIRT联合化疗对不可切除ICC患者的反应率。设计、设置和参与者 该 2 期临床试验,即 Yttrium-90 微球治疗胆管癌 (MISPHEC) 试验,纳入了从未接受过化疗或动脉内治疗且在 7 个有 SIRT 经验的中心接受治疗的不可切除 ICC 患者2013 年 11 月 12 日至 2016 年 6 月 21 日。统计分析时间为 2017 年 3 月 31 日至 2019 年 6 月 17 日。在 21 天周期的第 1 天和第 8 天,同时使用顺铂 25 mg/m2 和吉西他滨 1000 mg/m2 的一线化疗(在 SIRT 之前和之后的周期中,吉西他滨降低至 300 mg/m2) 8个周期。在第 1 周期(1 个半肝疾病)或第 1 和第 3 周期(涉及两个半肝的疾病)期间使用玻璃 Y90 微球进行选择性内部放射治疗。主要结果和措施 根据实体瘤反应评估标准 (RECIST) 1.1 在 3 个月时的反应率。根据 Choi 标准,次要终点是毒性作用、无进展生存期、总生存期、疾病控制率和缓解率。结果 纳入研究的 41 名患者中,26 名 (63%) 为男性,平均 (SD) 年龄为 64.0 (10.7) 岁。根据 RECIST 的反应率为 39%(90% CI,26%-53%)在 3 个月时根据当地审查确定,41% 被中央审查确认为最佳响应;疾病控制率为98%。根据Choi标准,响应率为93%。中位随访 36 个月(95% CI,26-52 个月)后,中位无进展生存期为 14 个月(95% CI,8-17 个月),12 岁时无进展生存率为 55%个月和 24 个月时为 30%。中位总生存期为 22 个月(95% CI,14-52 个月),12 个月和 24 个月的总生存率为 75% 和 45%。在 41 名患者中,29 名(71%)有 3 至 4 级毒性反应;9 名患者 (22%) 可降级为手术干预,其中 8 名 (20%) 达到 R0(无显微镜下切缘)手术切除。术后中位 46 个月(95% CI,31 个月至未达到)后,接受切除术的患者未达到中位无复发生存期。结论和相关性 联合化疗和 SIRT 作为不可切除 ICC 的一线治疗具有抗肿瘤活性,并且相当一部分患者降期至手术干预。3期试验正在进行中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号