Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Association Between Levothyroxine Treatment and Thyroid-Related Symptoms Among Adults Aged 80 Years and Older With Subclinical Hypothyroidism
JAMA ( IF 120.7 ) Pub Date : 2019-11-26 , DOI: 10.1001/jama.2019.17274 Simon P Mooijaart 1, 2 , Robert S Du Puy 3 , David J Stott 4 , Patricia M Kearney 5 , Nicolas Rodondi 6, 7 , Rudi G J Westendorp 8, 9 , Wendy P J den Elzen 10 , Iris Postmus 1, 2 , Rosalinde K E Poortvliet 3 , Diana van Heemst 1 , Barbara C van Munster 11 , Robin P Peeters 12 , Ian Ford 13 , Sharon Kean 13 , Claudia-Martina Messow 13 , Manuel R Blum 6, 14 , Tinh-Hai Collet 15 , Torquil Watt 16 , Olaf M Dekkers 17 , J Wouter Jukema 18 , Johannes W A Smit 19 , Peter Langhorne 20 , Jacobijn Gussekloo 1, 3
JAMA ( IF 120.7 ) Pub Date : 2019-11-26 , DOI: 10.1001/jama.2019.17274 Simon P Mooijaart 1, 2 , Robert S Du Puy 3 , David J Stott 4 , Patricia M Kearney 5 , Nicolas Rodondi 6, 7 , Rudi G J Westendorp 8, 9 , Wendy P J den Elzen 10 , Iris Postmus 1, 2 , Rosalinde K E Poortvliet 3 , Diana van Heemst 1 , Barbara C van Munster 11 , Robin P Peeters 12 , Ian Ford 13 , Sharon Kean 13 , Claudia-Martina Messow 13 , Manuel R Blum 6, 14 , Tinh-Hai Collet 15 , Torquil Watt 16 , Olaf M Dekkers 17 , J Wouter Jukema 18 , Johannes W A Smit 19 , Peter Langhorne 20 , Jacobijn Gussekloo 1, 3
Affiliation
|
Importance
It is unclear whether levothyroxine treatment provides clinically important benefits in adults aged 80 years and older with subclinical hypothyroidism. Objective
To determine the association of levothyroxine treatment for subclinical hypothyroidism with thyroid-related quality of life in adults aged 80 years and older. Design, Setting, and Participants
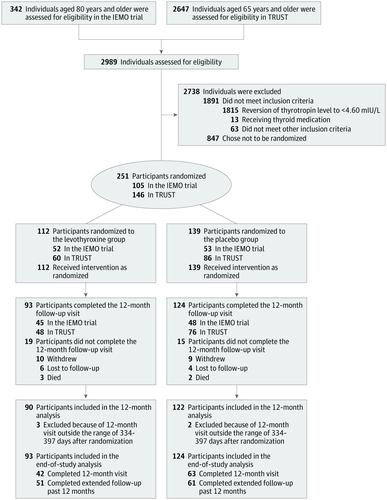
Prospectively planned combined analysis of data involving community-dwelling adults aged 80 years and older with subclinical hypothyroidism. Data from a randomized clinical trial were combined with a subgroup of participants aged 80 years and older from a second clinical trial. The trials were conducted between April 2013 and May 2018. Final follow-up was May 4, 2018. Exposures
Participants were randomly assigned to receive levothyroxine (n = 112; 52 participants from the first trial and 60 from the second trial) or placebo (n = 139; 53 participants from the first trial and 86 from the second trial). Main Outcomes and Measures
Co-primary outcomes were Thyroid-Related Quality of Life Patient-Reported Outcome (ThyPRO) questionnaire scores for the domains of hypothyroid symptoms and tiredness at 1 year (range, 0-100; higher scores indicate worse quality of life; minimal clinically important difference, 9). Results
Of 251 participants (mean age, 85 years; 118 [47%] women), 105 were included from the first clinical trial and 146 were included from the second clinical trial. A total of 212 participants (84%) completed the study. The hypothyroid symptoms score decreased from 21.7 at baseline to 19.3 at 12 months in the levothyroxine group vs from 19.8 at baseline to 17.4 at 12 months in the placebo group (adjusted between-group difference, 1.3 [95% CI, -2.7 to 5.2]; P = .53). The tiredness score increased from 25.5 at baseline to 28.2 at 12 months in the levothyroxine group vs from 25.1 at baseline to 28.7 at 12 months in the placebo group (adjusted between-group difference, -0.1 [95% CI, -4.5 to 4.3]; P = .96). At least 1 adverse event occurred in 33 participants (29.5%) in the levothyroxine group (the most common adverse event was cerebrovascular accident, which occurred in 3 participants [2.2%]) and 40 participants (28.8%) in the placebo group (the most common adverse event was pneumonia, which occurred in 4 [3.6%] participants). Conclusions and Relevance
In this prospectively planned analysis of data from 2 clinical trials involving adults aged 80 years and older with subclinical hypothyroidism, treatment with levothyroxine, compared with placebo, was not significantly associated with improvement in hypothyroid symptoms or fatigue. These findings do not support routine use of levothyroxine for treatment of subclinical hypothyroidism in adults aged 80 years and older. Trial Registration
ClinicalTrials.gov Identifier: NCT01660126; Netherlands Trial Register: NTR3851.
中文翻译:

80 岁及以上亚临床甲状腺功能减退症患者左旋甲状腺素治疗与甲状腺相关症状之间的关系
重要性 目前尚不清楚左旋甲状腺素治疗是否能为 80 岁及以上患有亚临床甲状腺功能减退症的成年人带来临床上重要的益处。目的 确定左旋甲状腺素治疗亚临床甲状腺功能减退症与 80 岁及以上成年人甲状腺相关生活质量的关系。设计、背景和参与者 前瞻性计划对涉及 80 岁及以上患有亚临床甲状腺功能减退症的社区居民的数据进行综合分析。来自一项随机临床试验的数据与来自第二项临床试验的一组 80 岁及以上的参与者亚组相结合。这些试验于 2013 年 4 月至 2018 年 5 月期间进行。最终随访时间为 2018 年 5 月 4 日。暴露 参与者被随机分配接受左甲状腺素治疗(n = 112;第一项试验 52 名参与者,第二项试验 60 名参与者)或安慰剂( n = 139;第一次试验有 53 名参与者,第二次试验有 86 名参与者)。主要结果和措施 共同主要结果是患者报告的甲状腺相关生活质量 (ThyPRO) 问卷评分,涉及 1 年甲状腺功能减退症状和疲劳领域(范围:0-100;分数越高表明生活质量越差;分数越高,表明生活质量越差。最小的临床重要差异,9)。结果 251 名参与者(平均年龄 85 岁;118 名女性 [47%])中,105 名来自第一次临床试验,146 名来自第二次临床试验。共有 212 名参与者 (84%) 完成了这项研究。左旋甲状腺素组的甲状腺功能减退症状评分从基线时的 21.7 降至 12 个月时的 19.3,而安慰剂组则从基线时的 19.8 降至 12 个月时的 17.4(调整后的组间差异为 1.3 [95% CI,-2.7 至 5.2]) ;P = .53)。左旋甲状腺素组的疲劳评分从基线时的 25.5 升至 12 个月时的 28.2,而安慰剂组则从基线时的 25.1 升至 12 个月时的 28.7(调整后的组间差异,-0.1 [95% CI,-4.5 至 4.3]) ;P = .96)。左旋甲状腺素组有 33 名参与者(29.5%)发生至少 1 次不良事件(最常见的不良事件是脑血管意外,有 3 名参与者发生 [2.2%]),安慰剂组有 40 名参与者(28.8%)发生至少 1 次不良事件(最常见的不良事件是脑血管意外)。最常见的不良事件是肺炎,发生在 4 名 [3.6%] 参与者中)。结论和相关性 在这项前瞻性计划分析中,对 2 项涉及 80 岁及以上患有亚临床甲状腺功能减退症成人的临床试验的数据进行了分析,结果显示,与安慰剂相比,左旋甲状腺素治疗与甲状腺功能减退症状或疲劳的改善没有显着相关性。这些发现并不支持常规使用左旋甲状腺素治疗 80 岁及以上成年人的亚临床甲状腺功能减退症。试验注册 ClinicalTrials.gov 标识符:NCT01660126;荷兰试用注册号:NTR3851。
更新日期:2019-11-26
中文翻译:

80 岁及以上亚临床甲状腺功能减退症患者左旋甲状腺素治疗与甲状腺相关症状之间的关系
重要性 目前尚不清楚左旋甲状腺素治疗是否能为 80 岁及以上患有亚临床甲状腺功能减退症的成年人带来临床上重要的益处。目的 确定左旋甲状腺素治疗亚临床甲状腺功能减退症与 80 岁及以上成年人甲状腺相关生活质量的关系。设计、背景和参与者 前瞻性计划对涉及 80 岁及以上患有亚临床甲状腺功能减退症的社区居民的数据进行综合分析。来自一项随机临床试验的数据与来自第二项临床试验的一组 80 岁及以上的参与者亚组相结合。这些试验于 2013 年 4 月至 2018 年 5 月期间进行。最终随访时间为 2018 年 5 月 4 日。暴露 参与者被随机分配接受左甲状腺素治疗(n = 112;第一项试验 52 名参与者,第二项试验 60 名参与者)或安慰剂( n = 139;第一次试验有 53 名参与者,第二次试验有 86 名参与者)。主要结果和措施 共同主要结果是患者报告的甲状腺相关生活质量 (ThyPRO) 问卷评分,涉及 1 年甲状腺功能减退症状和疲劳领域(范围:0-100;分数越高表明生活质量越差;分数越高,表明生活质量越差。最小的临床重要差异,9)。结果 251 名参与者(平均年龄 85 岁;118 名女性 [47%])中,105 名来自第一次临床试验,146 名来自第二次临床试验。共有 212 名参与者 (84%) 完成了这项研究。左旋甲状腺素组的甲状腺功能减退症状评分从基线时的 21.7 降至 12 个月时的 19.3,而安慰剂组则从基线时的 19.8 降至 12 个月时的 17.4(调整后的组间差异为 1.3 [95% CI,-2.7 至 5.2]) ;P = .53)。左旋甲状腺素组的疲劳评分从基线时的 25.5 升至 12 个月时的 28.2,而安慰剂组则从基线时的 25.1 升至 12 个月时的 28.7(调整后的组间差异,-0.1 [95% CI,-4.5 至 4.3]) ;P = .96)。左旋甲状腺素组有 33 名参与者(29.5%)发生至少 1 次不良事件(最常见的不良事件是脑血管意外,有 3 名参与者发生 [2.2%]),安慰剂组有 40 名参与者(28.8%)发生至少 1 次不良事件(最常见的不良事件是脑血管意外)。最常见的不良事件是肺炎,发生在 4 名 [3.6%] 参与者中)。结论和相关性 在这项前瞻性计划分析中,对 2 项涉及 80 岁及以上患有亚临床甲状腺功能减退症成人的临床试验的数据进行了分析,结果显示,与安慰剂相比,左旋甲状腺素治疗与甲状腺功能减退症状或疲劳的改善没有显着相关性。这些发现并不支持常规使用左旋甲状腺素治疗 80 岁及以上成年人的亚临床甲状腺功能减退症。试验注册 ClinicalTrials.gov 标识符:NCT01660126;荷兰试用注册号:NTR3851。


























 京公网安备 11010802027423号
京公网安备 11010802027423号