当前位置:
X-MOL 学术
›
Lancet Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval.
The Lancet Psychiatry ( IF 64.3 ) Pub Date : 2019-11-01 , DOI: 10.1016/s2215-0366(19)30394-3 Erick H Turner 1
The Lancet Psychiatry ( IF 64.3 ) Pub Date : 2019-11-01 , DOI: 10.1016/s2215-0366(19)30394-3 Erick H Turner 1
Affiliation
|
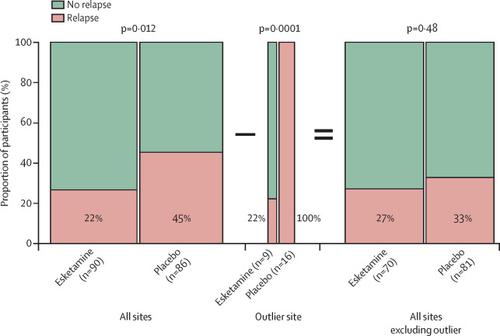
With a novel mechanism of action compared with existing marketed antidepressants, esketamine has been of keen interest to mental health clinicians and researchers. On March 5, 2019, the US Food and Drug Administration (FDA) approved intranasal esketamine for treatment-resistant depression. To make a proper risk–benefit analysis before prescribing, mental health clinicians should look beyond the fact of approval and consider the data from the phase 3 clinical trials, which are freely available in the public domain in the form of the slides and briefing documents from the Feb 12, 2019, Psychopharmacologic Drugs Advisory Committee. These documents reveal the following concerns regarding the safety of esketamine.
中文翻译:

Esketamine 治疗难治性抑郁症:关于疗效和 FDA 批准的七个问题。
与现有市售抗抑郁药相比,艾氯胺酮具有全新的作用机制,一直受到心理健康临床医生和研究人员的浓厚兴趣。2019 年 3 月 5 日,美国食品和药物管理局 (FDA) 批准鼻内艾氯胺酮用于难治性抑郁症。为了在开处方前进行适当的风险收益分析,心理健康临床医生应该超越批准的事实,并考虑来自 3 期临床试验的数据,这些数据可以在公共领域以幻灯片和简报文件的形式免费获得2019 年 2 月 12 日,精神药理学药物咨询委员会。这些文件揭示了以下关于艾氯胺酮安全性的担忧。
更新日期:2019-11-22
中文翻译:

Esketamine 治疗难治性抑郁症:关于疗效和 FDA 批准的七个问题。
与现有市售抗抑郁药相比,艾氯胺酮具有全新的作用机制,一直受到心理健康临床医生和研究人员的浓厚兴趣。2019 年 3 月 5 日,美国食品和药物管理局 (FDA) 批准鼻内艾氯胺酮用于难治性抑郁症。为了在开处方前进行适当的风险收益分析,心理健康临床医生应该超越批准的事实,并考虑来自 3 期临床试验的数据,这些数据可以在公共领域以幻灯片和简报文件的形式免费获得2019 年 2 月 12 日,精神药理学药物咨询委员会。这些文件揭示了以下关于艾氯胺酮安全性的担忧。


























 京公网安备 11010802027423号
京公网安备 11010802027423号