当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular Borylation via Sequential B-Mes Bond Cleavage for the Divergent Synthesis of B,N,B-Doped Benzo[4]helicenes.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-16 , DOI: 10.1002/anie.201912340 Julius A Knöller 1, 2 , Guoyun Meng 3 , Xiang Wang 1 , David Hall 4, 5 , Anton Pershin 5 , David Beljonne 5 , Yoann Olivier 6 , Sabine Laschat 2 , Eli Zysman-Colman 4 , Suning Wang 1, 3
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-16 , DOI: 10.1002/anie.201912340 Julius A Knöller 1, 2 , Guoyun Meng 3 , Xiang Wang 1 , David Hall 4, 5 , Anton Pershin 5 , David Beljonne 5 , Yoann Olivier 6 , Sabine Laschat 2 , Eli Zysman-Colman 4 , Suning Wang 1, 3
Affiliation
|
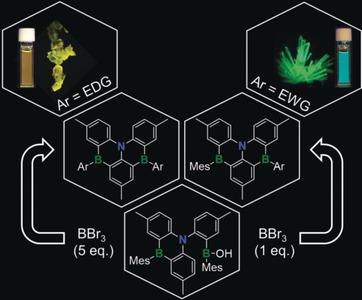
New symmetric and unsymmetric B,N,B-doped benzo[4]helicenes 3-6 a/b have been achieved in good yields, using a three-step process, starting from N(tolyl)3 in a highly divergent manner (7 examples). A borinic acid functionalized 1,4-B,N-anthracene 1 was found to display unprecedented reactivity, acting as a convenient and highly effective precursor for selective formation of bromo-substituted B,N,B-benzo[4]helicenes 2 a/2 b via intramolecular borylation and sequential B-Mes bond cleavage in the presence of BBr3 . Subsequent reaction of 2 a/2 b with Ar-Li provided a highly effective toolbox for the preparation of symmetrically/unsymmetrically functionalized B,N,B-helicenes. Their high photoluminescence quantum yields along with the small ΔEST suggest their potential as thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs).
中文翻译:

通过顺序B-Mes键断裂进行分子内硼化,以合成B,N,B掺杂的苯并[4]螺旋。
使用三步工艺,从N(甲苯基)3开始,以高度分散的方式,以高收率获得了新的对称和不对称的B,N,B掺杂的苯并[4]螺旋3-6 a / b(7例子)。发现硼酸官能化的1,4-B,N-蒽1显示出前所未有的反应活性,可作为选择性形成溴取代的B,N,B-苯并[4]螺旋烯2 a /的便捷高效前体。在存在BBr 3的情况下,通过分子内的硼酸化和顺序的B-Mes键裂解,图2b所示。随后2 a / 2 b与Ar-Li的反应为制备对称/不对称官能化的B,N,B-螺旋烯提供了一个高效的工具箱。
更新日期:2020-01-16
中文翻译:

通过顺序B-Mes键断裂进行分子内硼化,以合成B,N,B掺杂的苯并[4]螺旋。
使用三步工艺,从N(甲苯基)3开始,以高度分散的方式,以高收率获得了新的对称和不对称的B,N,B掺杂的苯并[4]螺旋3-6 a / b(7例子)。发现硼酸官能化的1,4-B,N-蒽1显示出前所未有的反应活性,可作为选择性形成溴取代的B,N,B-苯并[4]螺旋烯2 a /的便捷高效前体。在存在BBr 3的情况下,通过分子内的硼酸化和顺序的B-Mes键裂解,图2b所示。随后2 a / 2 b与Ar-Li的反应为制备对称/不对称官能化的B,N,B-螺旋烯提供了一个高效的工具箱。



























 京公网安备 11010802027423号
京公网安备 11010802027423号