Chem ( IF 23.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.chempr.2019.10.003 Matthew B. Minus , Aaron L. Featherston , Sooyun Choi , Sam C. King , Scott J. Miller , Eric V. Anslyn
|
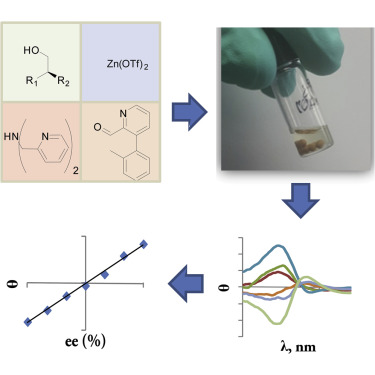
The use of parallel synthesis protocols for asymmetric reaction discovery has increased the need for new methods to rapidly determine enantiomeric excess (ee) values. Most chirality sensing is performed on stereocenters that are α (i.e., proximal) to the target functional group. Finding a general approach to detect more distant point chirality would increase the substrate scope of such assays. Herein, we demonstrate a design principle to “reach out” to more distant stereocenters, in this case β-chirality in primary alcohols. Therefore, we see the design principles established in this work as a step forward in sensing distant point chirality and, eventually, multi-stereocenter relationships.
中文翻译:

重新设计可逆的共价键组装体,以光学方式检测β-手性伯醇中的ee
使用平行合成方案进行不对称反应发现增加了对快速确定对映体过量(ee)值的新方法的需求。大多数手性感测是在距目标官能团α(即近端)的立体中心上进行的。寻找检测更远点手性的通用方法将增加此类测定的底物范围。本文中,我们展示了一种“延伸”到更远的立体中心的设计原理,在这种情况下,伯醇中的β-手性。因此,我们认为这项工作中确立的设计原则是在感测遥远点手性以及最终实现多立体中心关系方面迈出的一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号