当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PEGylation but Not Fc-Fusion Improves in Vivo Residence Time of a Thermostable Mutant of Bacterial Cocaine Esterase.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.bioconjchem.9b00622 Haifeng Huang 1, 2 , Lei Fang 2 , Liu Xue 2 , Ting Zhang 1, 2 , Kyungbo Kim 1, 2 , Shurong Hou 1, 2 , Fang Zheng 1, 2 , Chang-Guo Zhan 1, 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2019-11-25 , DOI: 10.1021/acs.bioconjchem.9b00622 Haifeng Huang 1, 2 , Lei Fang 2 , Liu Xue 2 , Ting Zhang 1, 2 , Kyungbo Kim 1, 2 , Shurong Hou 1, 2 , Fang Zheng 1, 2 , Chang-Guo Zhan 1, 2
Affiliation
|
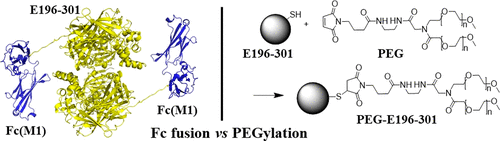
It is very popular to fuse a protein drug or drug candidate to the Fc domain of immunoglobulin G (IgG) in order to prolong the in vivo half-life. In this study, we have designed, prepared, and tested an Fc-fused thermostable cocaine esterase (CocE) mutant (known as E196-301, with the T172R/G173Q/L196C/I301C substitutions on CocE) expressed in E. coli. As expected, Fc-fusion does not affect the in vitro enzyme activity and thermal stability of the enzyme and that Fc-E196-301 can favorably bind FcRn with Kd = 386 ± 35 nM. However, Fc-fusion does not prolong the in vivo half-life of E196-301 at all; Fc-E196-301 and E196-301 have essentially the same PK profile (t1/2 = 0.4 ± 0.1 h) in rats. This is the first time demonstrating that Fc-fusion does not prolong in vivo half-life of a protein. This finding is consistent with the mechanistic understanding that E196-301 and Fc-E196-301 are all degraded primarily through rapid proteolysis in the body. The Fc fusion cannot protect E196-301 from the proteolysis in the body. Nevertheless, it has been demonstrated that PEGylation can effectively protect E196-301, as the PEGylated E196-301, i.e., PEG-E196-301, has a significantly prolonged in vivo half-life. It has also been demonstrated that both E196-301 and PEG-E196-301 have dose-dependent in vivo half-lives (e.g., 19.9 ± 6.4 h for the elimination t1/2 of 30 mg/kg PEG-E196-301), as the endogenous proteolytic enzymes responsible for proteolysis of E196-301 (PEGylated or not) are nearly saturated by the high plasma concentration produced by a high dose of E196-301 or PEG-E196-301.
中文翻译:

聚乙二醇化而非Fc融合改善了细菌可卡因酯酶的热稳定突变体的体内滞留时间。
为了延长体内半衰期,将蛋白药物或候选药物与免疫球蛋白G(IgG)的Fc结构域融合是非常普遍的。在这项研究中,我们设计,制备和测试了在大肠杆菌中表达的Fc融合热稳定可卡因酯酶(CocE)突变体(称为E196-301,在CocE上具有T172R / G173Q / L196C / I301C替代)。如预期的那样,Fc融合不会影响酶的体外酶活性和热稳定性,并且Fc-E196-301可以以Kd = 386±35 nM良好地结合FcRn。但是,Fc融合根本不会延长E196-301的体内半衰期;Fc-E196-301和E196-301在大鼠中具有相同的PK分布(t1 / 2 = 0.4±0.1 h)。这是首次证明Fc融合不会延长蛋白质的体内半衰期。该发现与以下机理理解一致:E196-301和Fc-E196-301均主要通过体内的快速蛋白水解而降解。Fc融合不能保护E196-301免受体内蛋白水解的影响。然而,已经证明聚乙二醇化可以有效地保护E196-301,因为聚乙二醇化的E196-301(即PEG-E196-301)具有显着延长的体内半衰期。还已经证明E196-301和PEG-E196-301均具有剂量依赖性的体内半衰期(例如,对于30 mg / kg PEG-E196-301的t1 / 2消除,为19.9±6.4小时),因为负责高剂量E196-301或PEG-E196-301产生的高血浆浓度使负责E196-301蛋白水解的内源蛋白水解酶(已聚乙二醇化或未聚乙二醇化)几乎饱和。
更新日期:2019-11-28
中文翻译:

聚乙二醇化而非Fc融合改善了细菌可卡因酯酶的热稳定突变体的体内滞留时间。
为了延长体内半衰期,将蛋白药物或候选药物与免疫球蛋白G(IgG)的Fc结构域融合是非常普遍的。在这项研究中,我们设计,制备和测试了在大肠杆菌中表达的Fc融合热稳定可卡因酯酶(CocE)突变体(称为E196-301,在CocE上具有T172R / G173Q / L196C / I301C替代)。如预期的那样,Fc融合不会影响酶的体外酶活性和热稳定性,并且Fc-E196-301可以以Kd = 386±35 nM良好地结合FcRn。但是,Fc融合根本不会延长E196-301的体内半衰期;Fc-E196-301和E196-301在大鼠中具有相同的PK分布(t1 / 2 = 0.4±0.1 h)。这是首次证明Fc融合不会延长蛋白质的体内半衰期。该发现与以下机理理解一致:E196-301和Fc-E196-301均主要通过体内的快速蛋白水解而降解。Fc融合不能保护E196-301免受体内蛋白水解的影响。然而,已经证明聚乙二醇化可以有效地保护E196-301,因为聚乙二醇化的E196-301(即PEG-E196-301)具有显着延长的体内半衰期。还已经证明E196-301和PEG-E196-301均具有剂量依赖性的体内半衰期(例如,对于30 mg / kg PEG-E196-301的t1 / 2消除,为19.9±6.4小时),因为负责高剂量E196-301或PEG-E196-301产生的高血浆浓度使负责E196-301蛋白水解的内源蛋白水解酶(已聚乙二醇化或未聚乙二醇化)几乎饱和。


























 京公网安备 11010802027423号
京公网安备 11010802027423号