当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scale-Up of the Manufacturing Process To Produce Docetaxel-Loaded mPEG-b-p(HPMA-Bz) Block Copolymer Micelles for Pharmaceutical Applications.
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.oprd.9b00387 Jaleesa Bresseleers 1, 2 , Mahsa Bagheri 3 , Gert Storm 3, 4 , Josbert M Metselaar 5 , Wim E Hennink 3 , Silvie A Meeuwissen 1 , Jan C M van Hest 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.oprd.9b00387 Jaleesa Bresseleers 1, 2 , Mahsa Bagheri 3 , Gert Storm 3, 4 , Josbert M Metselaar 5 , Wim E Hennink 3 , Silvie A Meeuwissen 1 , Jan C M van Hest 2
Affiliation
|
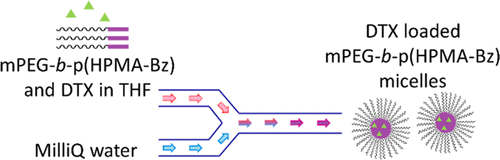
An efficient, scalable, and good manufacturing practice (GMP) compatible process was developed for the production of docetaxel-loaded poly(ethylene glycol)-b-poly(N-2-benzoyloxypropyl methacrylamide) (mPEG-b-p(HPMA-Bz)) micelles. First, the synthesis of the mPEG-b-p(HPMA-Bz) block copolymer was optimized through step-by-step investigation of the batch synthesis procedures. This resulted in the production of 1 kg of mPEG-b-p(HPMA-Bz) block copolymer with a 5 kDa PEG block and an overall molecular weight of 22.5 kDa. Second, the reproducibility and scalability of micelle formation was investigated for both batch and continuous flow setups by assessing critical process parameters. This resulted in the development of a new and highly efficient continuous flow process, which led to the production of 100 mL of unloaded micelles with a size of 55 nm. Finally, the loading of the micelles with the anticancer drug docetaxel was successfully fine-tuned to obtain precise control on the loaded micelle characteristics. As a result, 100 mL of docetaxel-loaded micelles (20 mg/mL polymer and 5 mg/mL docetaxel in the feed) with a size of 55 nm, an encapsulation efficiency of 65%, a loading capacity of 14%, and stable for at least 2 months in water at room temperature were produced with the newly developed continuous flow process. In conclusion, this study paves the way for efficient and robust large-scale production of docetaxel-loaded micelles with high encapsulation efficiencies and stability, which is crucial for their applicability as a clinically relevant drug delivery platform.
中文翻译:

扩大生产工艺以生产用于制药应用的多西他赛负载的mPEG-bp(HPMA-Bz)嵌段共聚物胶束。
开发了一种高效,可扩展且符合良好生产规范(GMP)的方法,用于生产负载紫杉杉醇的聚(乙二醇)-b-聚(N -2-苯甲酰氧基丙基甲基丙烯酰胺)(mPEG- b -p(HPMA-Bz ))胶束。首先,通过分批合成程序的逐步研究,优化了mPEG -b- p(HPMA-Bz)嵌段共聚物的合成。这样就产生了1公斤的mPEG- b-具有5 kDa PEG嵌段和22.5 kDa的总分子量的p(HPMA-Bz)嵌段共聚物。其次,通过评估关键工艺参数,研究了批量生产和连续生产中胶束形成的可重复性和可扩展性。这导致了新的高效连续流工艺的发展,这导致了100毫升大小为55 nm的卸载胶束的生产。最后,成功地微调了抗癌药多西他赛对胶束的负载,以精确控制负载的胶束特性。结果,负载100 mL多西紫杉醇的胶束(进料中20 mg / mL聚合物和5 mg / mL多西紫杉醇)的尺寸为55 nm,封装效率为65%,负载能力为14%,新开发的连续流工艺可在室温下在水中稳定至少2个月。总而言之,这项研究为高效,稳定地大规模生产多西他赛负载的胶束铺平了道路,该胶束具有很高的包封效率和稳定性,这对于它们作为临床相关药物递送平台的适用性至关重要。
更新日期:2019-11-13
中文翻译:

扩大生产工艺以生产用于制药应用的多西他赛负载的mPEG-bp(HPMA-Bz)嵌段共聚物胶束。
开发了一种高效,可扩展且符合良好生产规范(GMP)的方法,用于生产负载紫杉杉醇的聚(乙二醇)-b-聚(N -2-苯甲酰氧基丙基甲基丙烯酰胺)(mPEG- b -p(HPMA-Bz ))胶束。首先,通过分批合成程序的逐步研究,优化了mPEG -b- p(HPMA-Bz)嵌段共聚物的合成。这样就产生了1公斤的mPEG- b-具有5 kDa PEG嵌段和22.5 kDa的总分子量的p(HPMA-Bz)嵌段共聚物。其次,通过评估关键工艺参数,研究了批量生产和连续生产中胶束形成的可重复性和可扩展性。这导致了新的高效连续流工艺的发展,这导致了100毫升大小为55 nm的卸载胶束的生产。最后,成功地微调了抗癌药多西他赛对胶束的负载,以精确控制负载的胶束特性。结果,负载100 mL多西紫杉醇的胶束(进料中20 mg / mL聚合物和5 mg / mL多西紫杉醇)的尺寸为55 nm,封装效率为65%,负载能力为14%,新开发的连续流工艺可在室温下在水中稳定至少2个月。总而言之,这项研究为高效,稳定地大规模生产多西他赛负载的胶束铺平了道路,该胶束具有很高的包封效率和稳定性,这对于它们作为临床相关药物递送平台的适用性至关重要。

























 京公网安备 11010802027423号
京公网安备 11010802027423号