当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In vitro and in vivo toxicity evaluation of non-neuroleptic phenothiazines, antitubercular drug candidates.
Regulatory Toxicology and Pharmacology ( IF 3.4 ) Pub Date : 2019-10-28 , DOI: 10.1016/j.yrtph.2019.104508 Sumayah Salie 1 , Antoinette Labuschagné 1 , Avril Walters 1 , Sohair Geyer 1 , Anwar Jardine 2 , Muazzam Jacobs 3 , Nai-Jen Hsu 1
Regulatory Toxicology and Pharmacology ( IF 3.4 ) Pub Date : 2019-10-28 , DOI: 10.1016/j.yrtph.2019.104508 Sumayah Salie 1 , Antoinette Labuschagné 1 , Avril Walters 1 , Sohair Geyer 1 , Anwar Jardine 2 , Muazzam Jacobs 3 , Nai-Jen Hsu 1
Affiliation
|
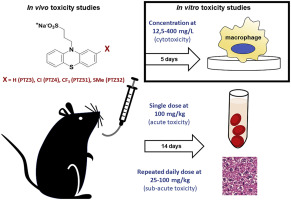
The phenothiazine-derived antipsychotic drugs, such as chlorpromazine and thioridazine, are bactericidal against drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis, but produce undesirable side effects at clinically relevant doses. We have previously modified four novel phenothiazines and maintained their antimycobacterial activity. This study evaluated the pharmacological and toxicity profiles of these novel non-neuroleptic phenothiazines, PTZ3, PTZ4, PTZ31 and PTZ32, for their metabolic stability, kinetic solubility and potential cytotoxic effects in vitro. To further support the safet use of these drug candidates, the in vivo pharmacological and toxicity profiles were assessed in C57BL/6 mice via single or repeated oral gavage. In acute toxicity studies, all four modified phenothiazines showed favourable safety in mice. When treated daily with 100 mg/kg of PTZ3 and PTZ4 for 2 weeks, mice displayed no signs of toxicity. Alternatively, treatment with PTZ31 resulted in 20% mortality with no toxicity evident in biochemical or histological analysis, while exposure to PTZ32 resulted in a 45% survival with increased serum concentrations of uric acid and alkaline phosphatase. The combined non-neuroleptic and antimycobacterial effects of the novel phenothiazines PTZ3, PTZ4, PTZ31 and PTZ32 demonstrated favourable pharmacological and toxicity profiles in this study, highlight the potential of these compounds as suitable anti-tuberculosis drug candidates.
中文翻译:

非抗精神病性吩噻嗪类抗结核药物候选药物的体外和体内毒性评估。
吩噻嗪衍生的抗精神病药,例如氯丙嗪和硫代哒嗪,对结核分枝杆菌的药物敏感和耐药菌株具有杀菌作用,但在临床相关剂量下会产生不良副作用。我们以前已经修改了四个新颖的吩噻嗪,并保持了它们的抗分枝杆菌活性。这项研究评估了这些新型非神经痛性吩噻嗪,PTZ3,PTZ4,PTZ31和PTZ32的药理学和毒性概况,了解它们在体外的代谢稳定性,动力学溶解度和潜在的细胞毒性作用。为了进一步支持这些候选药物的安全使用,通过一次或多次口服强饲法对C57BL / 6小鼠体内的药理和毒性进行了评估。在急性毒性研究中,所有四种修饰的吩噻嗪在小鼠中均显示出良好的安全性。每天用100 mg / kg的PTZ3和PTZ4处理2周时,小鼠未显示毒性迹象。另外,用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中没有明显的毒性,而暴露于PTZ32可使血清浓度的尿酸和碱性磷酸酶的存活率提高到45%。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在这项研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中没有明显的毒性,而暴露于PTZ32则可通过血清尿酸和碱性磷酸酶浓度的增加获得45%的存活率。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在本研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中无明显毒性;而暴露于PTZ32则可导致45%的生存率,且血清尿酸和碱性磷酸酶浓度升高。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在这项研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。
更新日期:2019-10-28
中文翻译:

非抗精神病性吩噻嗪类抗结核药物候选药物的体外和体内毒性评估。
吩噻嗪衍生的抗精神病药,例如氯丙嗪和硫代哒嗪,对结核分枝杆菌的药物敏感和耐药菌株具有杀菌作用,但在临床相关剂量下会产生不良副作用。我们以前已经修改了四个新颖的吩噻嗪,并保持了它们的抗分枝杆菌活性。这项研究评估了这些新型非神经痛性吩噻嗪,PTZ3,PTZ4,PTZ31和PTZ32的药理学和毒性概况,了解它们在体外的代谢稳定性,动力学溶解度和潜在的细胞毒性作用。为了进一步支持这些候选药物的安全使用,通过一次或多次口服强饲法对C57BL / 6小鼠体内的药理和毒性进行了评估。在急性毒性研究中,所有四种修饰的吩噻嗪在小鼠中均显示出良好的安全性。每天用100 mg / kg的PTZ3和PTZ4处理2周时,小鼠未显示毒性迹象。另外,用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中没有明显的毒性,而暴露于PTZ32可使血清浓度的尿酸和碱性磷酸酶的存活率提高到45%。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在这项研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中没有明显的毒性,而暴露于PTZ32则可通过血清尿酸和碱性磷酸酶浓度的增加获得45%的存活率。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在本研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。用PTZ31进行治疗可导致20%的死亡率,而在生化或组织学分析中无明显毒性;而暴露于PTZ32则可导致45%的生存率,且血清尿酸和碱性磷酸酶浓度升高。新型吩噻嗪类PTZ3,PTZ4,PTZ31和PTZ32的非神经毒性和抗分枝杆菌作用相结合,在这项研究中显示出良好的药理和毒性特征,突出了这些化合物作为合适的抗结核药物候选物的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号