当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unconventional Reactivity of Ethynylbenziodoxolone Reagents and Thiols: Scope and Mechanism.
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-22 , DOI: 10.1002/chem.201904520 Bin Liu 1 , Juan V Alegre-Requena 1 , Robert S Paton 1, 2 , Garret M Miyake 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-22 , DOI: 10.1002/chem.201904520 Bin Liu 1 , Juan V Alegre-Requena 1 , Robert S Paton 1, 2 , Garret M Miyake 1
Affiliation
|
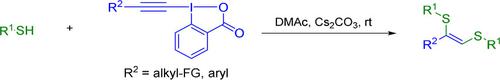
1,2-Dithio-1-alkenes are biologically active compounds widely implemented throughout organic synthesis, functional materials, coordination chemistry, and pharmaceuticals. Traditional methods for accessing 1,2-dithio-1-alkenes often demand transition metal catalysts, specialized or air-sensitive ligands, high temperatures, and disulfides (R2 S2 ). Herein, a general and efficient strategy utilizing ethynylbenziodoxolone (EBX) reagents and thiols is presented that results in the formation of 1,2-dithio-1-alkenes with excellent regioselectivity and stereoselectivity through unprecedented reactivity between the EBX and the thiol. This operationally simple procedure utilizes mild conditions, which result in a broad substrate scope and high functional-group tolerance. The observed unexpected reactivity has been rationalized through both experimental results and DFT calculations.
中文翻译:

乙炔基苯并氧杂环酮试剂和硫醇的非常规反应性:范围和机制。
1,2-二硫-1-烯烃是具有生物活性的化合物,广泛应用于有机合成、功能材料、配位化学和制药领域。获取 1,2-二硫代-1-烯烃的传统方法通常需要过渡金属催化剂、专用或空气敏感配体、高温和二硫化物 (R2 S2 )。在此,提出了一种利用乙炔基苯并氧杂环酮 (EBX) 试剂和硫醇的通用且有效的策略,通过 EBX 和硫醇之间前所未有的反应性,形成具有优异区域选择性和立体选择性的 1,2-二硫-1-烯烃。这种操作简单的程序利用温和的条件,从而产生广泛的底物范围和高官能团耐受性。通过实验结果和 DFT 计算,观察到的意外反应性已得到合理化。
更新日期:2020-01-23
中文翻译:

乙炔基苯并氧杂环酮试剂和硫醇的非常规反应性:范围和机制。
1,2-二硫-1-烯烃是具有生物活性的化合物,广泛应用于有机合成、功能材料、配位化学和制药领域。获取 1,2-二硫代-1-烯烃的传统方法通常需要过渡金属催化剂、专用或空气敏感配体、高温和二硫化物 (R2 S2 )。在此,提出了一种利用乙炔基苯并氧杂环酮 (EBX) 试剂和硫醇的通用且有效的策略,通过 EBX 和硫醇之间前所未有的反应性,形成具有优异区域选择性和立体选择性的 1,2-二硫-1-烯烃。这种操作简单的程序利用温和的条件,从而产生广泛的底物范围和高官能团耐受性。通过实验结果和 DFT 计算,观察到的意外反应性已得到合理化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号