当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and validation of a method to confirm the exogenous origin of prednisone and prednisolone by GC-C-IRMS.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2019-12-19 , DOI: 10.1002/dta.2715 Loredana Iannella 1, 2 , Francesco Botrè 1, 3 , Cristiana Colamonici 1 , Davide Curcio 1 , Xavier de la Torre 1
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2019-12-19 , DOI: 10.1002/dta.2715 Loredana Iannella 1, 2 , Francesco Botrè 1, 3 , Cristiana Colamonici 1 , Davide Curcio 1 , Xavier de la Torre 1
Affiliation
|
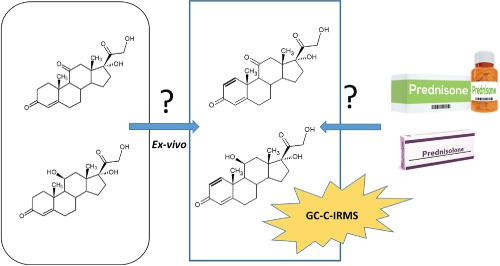
Prednisone and prednisolone are two anti‐inflammatory steroidal drugs listed by the World Anti‐Doping Agency (WADA) within the class of glucocorticoids, which are prohibited “in competition” and when administered systemically. Their presence in collected urine samples may be attributed, if no exogenous administration has occurred, to an in situ microbial formation from endogenous steroids. In this work, a gas chromatography coupled to carbon isotope ratio mass spectrometry (GC‐C‐IRMS) method was developed and validated to distinguish an exogenous origin from an endogenous one. Eight prednisone/prednisolone pharmaceutical preparations commercially available in Italy were analysed to establish an exogenous δ13C value reference range (−28.96 ± 0.39‰). No more than 25 mL of urine was processed and no derivatization nor intentional steroids structure modifications were performed before the GC‐C‐IRMS analysis. A first HPLC purification step was set up to isolate the three endogenous reference compounds (ERCs) selected (tetrahydro‐11‐deoxycortisol (THS), pregnanediol (PD), and pregnanetriol (PT)), while a second LC purification was necessary to separate prednisone from prednisolone. In the GC‐C‐IRMS analysis, two different GC run methods were set up to guarantee better sensitivity and selectivity for each compound. Both prednisone and prednisolone showed signals (m/z 44) with amplitudes within the method linearity range to a lower urinary concentration of 20 ng/mL (< WADA reporting level, 30 ng/mL). The method was fully validated according to WADA requirements. As a proof of concept, urine samples collected from two excretion studies in healthy male volunteers, after a prednisone or prednisolone administration, were analysed by the proposed method, demonstrating its applicability for the analysis of real samples.
中文翻译:

通过GC-C-IRMS确认强的松和泼尼松龙的外源性方法的开发和验证。
泼尼松和泼尼松龙是世界反兴奋剂机构(WADA)在糖皮质激素类中列出的两种抗炎类固醇药物,在竞争中和系统给药时均被禁止。如果未进行外源性给药,则它们在收集的尿液样本中的存在可能归因于内源性类固醇的原位微生物形成。在这项工作中,开发了一种气相色谱与碳同位素比质谱法(GC-C-IRMS)的方法,并进行了验证,以区分外源与内源。八泼尼松/泼尼松龙药物制剂在意大利市售进行分析,以建立一个外源性δ 13C值参考范围(−28.96±0.39‰)。在进行GC‐C‐IRMS分析之前,处理的尿液量不超过25 mL,没有进行衍生化处理或有意的类固醇结构修饰。设置了第一个HPLC纯化步骤以分离选择的三种内源参考化合物(ERC)(四氢-11-脱氧皮质醇(THS),孕烯二醇(PD)和孕烯醇(PT)),而第二次LC纯化则需要分离泼尼松龙的泼尼松。在GC-C-IRMS分析中,设置了两种不同的气相色谱运行方法,以确保每种化合物具有更好的灵敏度和选择性。泼尼松和泼尼松龙均显示信号(m / z 44),幅度在方法线性范围内,尿液浓度较低,为20 ng / mL(<WADA报告水平,为30 ng / mL)。该方法已根据WADA要求进行了充分验证。作为概念的证明,在泼尼松或泼尼松龙给药后,从健康男性志愿者的两次排泄研究中收集的尿液样品通过提出的方法进行了分析,证明了其在实际样品分析中的适用性。
更新日期:2019-12-19
中文翻译:

通过GC-C-IRMS确认强的松和泼尼松龙的外源性方法的开发和验证。
泼尼松和泼尼松龙是世界反兴奋剂机构(WADA)在糖皮质激素类中列出的两种抗炎类固醇药物,在竞争中和系统给药时均被禁止。如果未进行外源性给药,则它们在收集的尿液样本中的存在可能归因于内源性类固醇的原位微生物形成。在这项工作中,开发了一种气相色谱与碳同位素比质谱法(GC-C-IRMS)的方法,并进行了验证,以区分外源与内源。八泼尼松/泼尼松龙药物制剂在意大利市售进行分析,以建立一个外源性δ 13C值参考范围(−28.96±0.39‰)。在进行GC‐C‐IRMS分析之前,处理的尿液量不超过25 mL,没有进行衍生化处理或有意的类固醇结构修饰。设置了第一个HPLC纯化步骤以分离选择的三种内源参考化合物(ERC)(四氢-11-脱氧皮质醇(THS),孕烯二醇(PD)和孕烯醇(PT)),而第二次LC纯化则需要分离泼尼松龙的泼尼松。在GC-C-IRMS分析中,设置了两种不同的气相色谱运行方法,以确保每种化合物具有更好的灵敏度和选择性。泼尼松和泼尼松龙均显示信号(m / z 44),幅度在方法线性范围内,尿液浓度较低,为20 ng / mL(<WADA报告水平,为30 ng / mL)。该方法已根据WADA要求进行了充分验证。作为概念的证明,在泼尼松或泼尼松龙给药后,从健康男性志愿者的两次排泄研究中收集的尿液样品通过提出的方法进行了分析,证明了其在实际样品分析中的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号