当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monomeric and homotrimeric solution structures of truncated human peroxidasin 1 variants.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2019-07-08 , DOI: 10.1016/j.bbapap.2019.07.002 Martina Paumann-Page 1 , Rupert Tscheliessnig 2 , Benjamin Sevcnikar 1 , Romy-Sophie Katz 1 , Irene Schwartz 1 , Stefan Hofbauer 1 , Vera Pfanzagl 1 , Paul G Furtmüller 1 , Christian Obinger 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2019-07-08 , DOI: 10.1016/j.bbapap.2019.07.002 Martina Paumann-Page 1 , Rupert Tscheliessnig 2 , Benjamin Sevcnikar 1 , Romy-Sophie Katz 1 , Irene Schwartz 1 , Stefan Hofbauer 1 , Vera Pfanzagl 1 , Paul G Furtmüller 1 , Christian Obinger 1
Affiliation
|
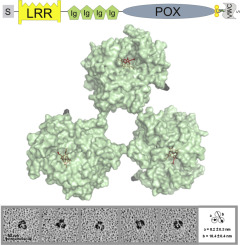
Human peroxidasin 1 is a multidomain peroxidase situated in the basement membrane. The iron enzyme with covalently bound heme oxidizes bromide to hypobromous acid which facilitates the formation of distinct sulfilimine cross-links in the collagen IV network and therefore contributes to its mechanical stability. Additional to the catalytically active peroxidase domain peroxidasin comprises a leucine rich repeat domain, four Ig domains and a C-terminal von Willebrand factor type C module (VWC). Peroxidasin has been shown to form homotrimers involving two redox-sensitive cysteine residues and to undergo posttranslational C-terminal proteolytic cleavage. The present study on several recombinantly produced truncated peroxidasin variants showed that the VWC is not required for trimer formation whereas the alpha-helical linker region located between the peroxidase domain and the VWC is crucial for trimerization. Our data furthermore implies that peroxidasin oligomerization occurs intracellularly before C-terminal cleavage. For the first time we present overall solution structures of monomeric and trimeric truncated peroxidasin variants which were determined by rotary shadowing combined with transmission electron microscopy and by small-angle X-ray scattering (SAXS). A triangular arrangement of the peroxidase domains to each other within the homotrimer was revealed and this structure was confirmed by a model of trimeric peroxidase domains. Our SAXS data showed that the Ig domains are highly flexible and interact with the peroxidase domain and that within the homotrimer each alpha-helical linker region interacts with the respective adjacent peroxidase domain. The implications of our findings on the structure-function relationship of peroxidasin are discussed.
中文翻译:

截短的人过氧化物酶 1 变体的单体和同源三聚体溶液结构。
人过氧化物酶 1 是位于基底膜中的多结构域过氧化物酶。具有共价结合血红素的铁酶将溴化物氧化为次溴酸,这有助于在 IV 型胶原网络中形成不同的硫亚胺交联,因此有助于其机械稳定性。除了具有催化活性的过氧化物酶结构域外,过氧化物酶还包括一个富含亮氨酸的重复结构域、四个 Ig 结构域和一个 C 端血管性血友病因子 C 型模块 (VWC)。Peroxidasin 已显示形成涉及两个氧化还原敏感半胱氨酸残基的同源三聚体,并经历翻译后 C 末端蛋白水解切割。目前对几种重组产生的截短过氧化物酶变体的研究表明,VWC 不是三聚体形成所必需的,而位于过氧化物酶结构域和 VWC 之间的 α-螺旋接头区域对于三聚化至关重要。我们的数据进一步暗示过氧化物酶寡聚在 C 末端裂解之前发生在细胞内。我们首次展示了单体和三聚体截短的过氧化物酶变体的整体溶液结构,这些结构是通过旋转阴影结合透射电子显微镜和小角 X 射线散射 (SAXS) 确定的。揭示了同源三聚体中过氧化物酶结构域彼此之间的三角形排列,并且这种结构通过三聚体过氧化物酶结构域的模型得到证实。我们的 SAXS 数据显示,Ig 结构域高度灵活并与过氧化物酶结构域相互作用,并且在同源三聚体中,每个 α-螺旋接头区域与各自相邻的过氧化物酶结构域相互作用。讨论了我们的研究结果对过氧化物酶的结构-功能关系的影响。
更新日期:2019-10-25
中文翻译:

截短的人过氧化物酶 1 变体的单体和同源三聚体溶液结构。
人过氧化物酶 1 是位于基底膜中的多结构域过氧化物酶。具有共价结合血红素的铁酶将溴化物氧化为次溴酸,这有助于在 IV 型胶原网络中形成不同的硫亚胺交联,因此有助于其机械稳定性。除了具有催化活性的过氧化物酶结构域外,过氧化物酶还包括一个富含亮氨酸的重复结构域、四个 Ig 结构域和一个 C 端血管性血友病因子 C 型模块 (VWC)。Peroxidasin 已显示形成涉及两个氧化还原敏感半胱氨酸残基的同源三聚体,并经历翻译后 C 末端蛋白水解切割。目前对几种重组产生的截短过氧化物酶变体的研究表明,VWC 不是三聚体形成所必需的,而位于过氧化物酶结构域和 VWC 之间的 α-螺旋接头区域对于三聚化至关重要。我们的数据进一步暗示过氧化物酶寡聚在 C 末端裂解之前发生在细胞内。我们首次展示了单体和三聚体截短的过氧化物酶变体的整体溶液结构,这些结构是通过旋转阴影结合透射电子显微镜和小角 X 射线散射 (SAXS) 确定的。揭示了同源三聚体中过氧化物酶结构域彼此之间的三角形排列,并且这种结构通过三聚体过氧化物酶结构域的模型得到证实。我们的 SAXS 数据显示,Ig 结构域高度灵活并与过氧化物酶结构域相互作用,并且在同源三聚体中,每个 α-螺旋接头区域与各自相邻的过氧化物酶结构域相互作用。讨论了我们的研究结果对过氧化物酶的结构-功能关系的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号