当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
G-quadruplexes Sequester Free Heme in Living Cells.
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2019-10-23 , DOI: 10.1016/j.chembiol.2019.10.003 Lucas T Gray 1 , Emilia Puig Lombardi 2 , Daniela Verga 3 , Alain Nicolas 2 , Marie-Paule Teulade-Fichou 3 , Arturo Londoño-Vallejo 2 , Nancy Maizels 4
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2019-10-23 , DOI: 10.1016/j.chembiol.2019.10.003 Lucas T Gray 1 , Emilia Puig Lombardi 2 , Daniela Verga 3 , Alain Nicolas 2 , Marie-Paule Teulade-Fichou 3 , Arturo Londoño-Vallejo 2 , Nancy Maizels 4
Affiliation
|
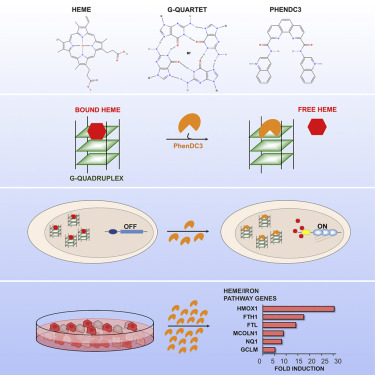
Heme is an essential cofactor for many enzymes, but free heme is toxic and its levels are tightly regulated. G-quadruplexes bind heme avidly in vitro, raising the possibility that they may sequester heme in vivo. If so, then treatment that displaces heme from quadruplexes is predicted to induce expression of genes involved in iron and heme homeostasis. Here we show that PhenDC3, a G-quadruplex ligand structurally unrelated to heme, displaces quadruplex-bound heme in vitro and alters transcription in cultured human cells, upregulating genes that support heme degradation and iron homeostasis, and most strikingly causing a 30-fold induction of heme oxidase 1, the key enzyme in heme degradation. We propose that G-quadruplexes sequester heme to protect cells from the pathophysiological consequences of free heme.
中文翻译:

G-四链体隔离活细胞中的游离血红素。
血红素是许多酶的重要辅助因子,但游离血红素是有毒的,其水平受到严格调控。G-四链体在体外强烈结合血红素,增加了它们在体内隔离血红素的可能性。如果是这样,那么从四链体置换血红素的治疗预计会诱导参与铁和血红素稳态的基因的表达。在这里我们展示了 PhenDC3,一种在结构上与血红素无关的 G-四链体配体,在体外取代四链体结合的血红素并改变培养的人类细胞中的转录,上调支持血红素降解和铁稳态的基因,最引人注目的是引起 30 倍的诱导血红素氧化酶 1,血红素降解的关键酶。我们建议 G-四链体隔离血红素以保护细胞免受游离血红素的病理生理后果。
更新日期:2019-11-09
中文翻译:

G-四链体隔离活细胞中的游离血红素。
血红素是许多酶的重要辅助因子,但游离血红素是有毒的,其水平受到严格调控。G-四链体在体外强烈结合血红素,增加了它们在体内隔离血红素的可能性。如果是这样,那么从四链体置换血红素的治疗预计会诱导参与铁和血红素稳态的基因的表达。在这里我们展示了 PhenDC3,一种在结构上与血红素无关的 G-四链体配体,在体外取代四链体结合的血红素并改变培养的人类细胞中的转录,上调支持血红素降解和铁稳态的基因,最引人注目的是引起 30 倍的诱导血红素氧化酶 1,血红素降解的关键酶。我们建议 G-四链体隔离血红素以保护细胞免受游离血红素的病理生理后果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号