Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into ceNAP1 Chaperoning Activity toward ceH2A-H2B.
Structure ( IF 5.7 ) Pub Date : 2019-10-22 , DOI: 10.1016/j.str.2019.10.002 Yongrui Liu 1 , Li Xu 1 , Changlin Xie 1 , Jingjun Hong 1 , Fudong Li 1 , Ke Ruan 1 , Jiajing Chen 1 , Jihui Wu 1 , Yunyu Shi 1
Structure ( IF 5.7 ) Pub Date : 2019-10-22 , DOI: 10.1016/j.str.2019.10.002 Yongrui Liu 1 , Li Xu 1 , Changlin Xie 1 , Jingjun Hong 1 , Fudong Li 1 , Ke Ruan 1 , Jiajing Chen 1 , Jihui Wu 1 , Yunyu Shi 1
Affiliation
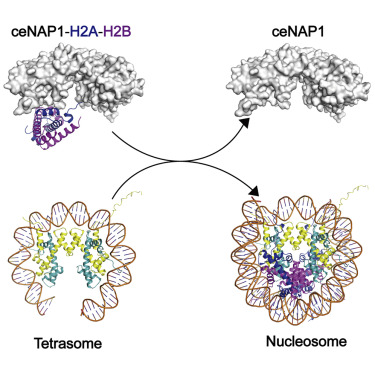
|
In eukaryotes, nucleosome assembly is crucial for genome integrity. The histone chaperone NAP1 plays an important role in histone folding, storage, and transport, as well as histone exchange and nucleosome assembly. At present, the molecular basis of these activities is not fully understood. We have solved high-resolution crystal structures of Caenorhabditis elegans NAP1 (ceNAP1) in complex with its cognate substrates: the C. elegans H2A-H2B dimer (ceH2A-H2B) and the H2A.Z-H2B dimer (ceH2A.Z-H2B). Our structural and biochemical data reveals the acidic concave surface is relevant to tetramerization, and uncovers how a ceNAP1 homodimer uses its concave surface to asymmetrically recognize a ceH2A-H2B or ceH2A.Z-H2B heterodimer. Intriguingly, an "acidic strip" within the concave surface of ceNAP1 is crucial for binding histones, including H2A-H2B, H3-H4, and histone variants. Thus, our results provide insight into the molecular mechanisms of NAP1 histone chaperone activity.
中文翻译:

ceNAP1对ceH2A-H2B的伴侣活动的结构见解。
在真核生物中,核小体组装对于基因组完整性至关重要。组蛋白伴侣NAP1在组蛋白折叠,存储和运输以及组蛋白交换和核小体组装中起重要作用。目前,尚未完全了解这些活性的分子基础。我们已经解决了秀丽隐杆线虫NAP1(ceNAP1)及其同源底物的复杂晶体结构:秀丽隐杆线虫H2A-H2B二聚体(ceH2A-H2B)和H2A.Z-H2B二聚体(ceH2A.Z-H2B) 。我们的结构和生化数据表明,酸性凹面与四聚化有关,并揭示了ceNAP1同型二聚体如何利用其凹面不对称地识别ceH2A-H2B或ceH2A.Z-H2B异二聚体。有趣的是,ceNAP1凹面内的“酸性条”对于结合组蛋白至关重要,包括H2A-H2B,H3-H4和组蛋白变体。因此,我们的结果提供了NAP1组蛋白伴侣分子活性的分子机制的见解。
更新日期:2019-10-23
中文翻译:

ceNAP1对ceH2A-H2B的伴侣活动的结构见解。
在真核生物中,核小体组装对于基因组完整性至关重要。组蛋白伴侣NAP1在组蛋白折叠,存储和运输以及组蛋白交换和核小体组装中起重要作用。目前,尚未完全了解这些活性的分子基础。我们已经解决了秀丽隐杆线虫NAP1(ceNAP1)及其同源底物的复杂晶体结构:秀丽隐杆线虫H2A-H2B二聚体(ceH2A-H2B)和H2A.Z-H2B二聚体(ceH2A.Z-H2B) 。我们的结构和生化数据表明,酸性凹面与四聚化有关,并揭示了ceNAP1同型二聚体如何利用其凹面不对称地识别ceH2A-H2B或ceH2A.Z-H2B异二聚体。有趣的是,ceNAP1凹面内的“酸性条”对于结合组蛋白至关重要,包括H2A-H2B,H3-H4和组蛋白变体。因此,我们的结果提供了NAP1组蛋白伴侣分子活性的分子机制的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号