当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective conversion of chitin to levulinic acid catalyzed by ionic liquids: distinctive effect of N-acetyl groups†
Green Chemistry ( IF 9.8 ) Pub Date : 2019-10-21 , DOI: 10.1039/c9gc02669j Wuxin Hou 1, 2, 3, 4 , Qingyang Zhao 1, 2, 3, 4 , Li Liu 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2019-10-21 , DOI: 10.1039/c9gc02669j Wuxin Hou 1, 2, 3, 4 , Qingyang Zhao 1, 2, 3, 4 , Li Liu 1, 2, 3, 4
Affiliation
|
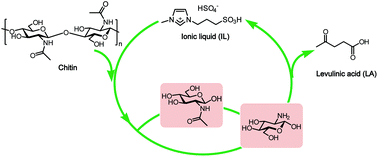
Selective and green conversion of chitin to levulinic acid (LA) has been realized by the catalysis of the ionic liquid (IL) 1-methyl-3-(3-sulfopropyl)imidazolium hydrogen sulfate ([C3SO3Hmim]HSO4) up to a yield of 67.0%. The relationship between the structure of IL and the yield of LA was investigated, demonstrating that both acidity and the hydrogen bonding ability of IL dictate its catalytic activity. Furthermore, by means of NMR, IR, SEM, GPC analyses and determination of the degree of acetylation (DA), two approaches of deacetylation–depolymerization to glucosamine (GlcN) and direct depolymerization to N-acetylglucosamine (GlcNAc) were proposed as chitin hydrolysis mechanisms, which occur only at the outer surface while leaving the interior crystalline chitin intact. The N-acetyl groups in chitin construct strong intramolecular and intermolecular hydrogen bond networks that contribute to the integration of the crystalline structure and building up of barriers to shield the accessibility of glycosidic linkages with the acidic catalyst.
中文翻译:

离子液体催化甲壳素选择性转化为乙酰丙酸:N-乙酰基的独特作用†
通过催化离子液体(IL)1-甲基-3-(3-磺丙基)咪唑硫酸氢盐([C 3 SO 3 Hmim] HSO 4)实现了几丁质向乙酰丙酸(LA)的选择性绿色转化。产率高达67.0%。研究了IL的结构与LA的产率之间的关系,表明IL的酸度和氢键结合能力决定了其催化活性。此外,借助NMR,IR,SEM,GPC分析和确定乙酰化度(DA),有两种脱乙酰方法-解聚为氨基葡萄糖(GlcN)和直接解聚为N有人建议使用-乙酰基葡糖胺(GlcNAc)作为几丁质的水解机理,这种酶仅在外表面发生,而内部的结晶几丁质保持完好无损。几丁质中的N-乙酰基构成了强大的分子内和分子间氢键网络,这些网络有助于晶体结构的整合和屏障的形成,从而屏蔽了糖苷键与酸性催化剂的可及性。
更新日期:2020-01-02
中文翻译:

离子液体催化甲壳素选择性转化为乙酰丙酸:N-乙酰基的独特作用†
通过催化离子液体(IL)1-甲基-3-(3-磺丙基)咪唑硫酸氢盐([C 3 SO 3 Hmim] HSO 4)实现了几丁质向乙酰丙酸(LA)的选择性绿色转化。产率高达67.0%。研究了IL的结构与LA的产率之间的关系,表明IL的酸度和氢键结合能力决定了其催化活性。此外,借助NMR,IR,SEM,GPC分析和确定乙酰化度(DA),有两种脱乙酰方法-解聚为氨基葡萄糖(GlcN)和直接解聚为N有人建议使用-乙酰基葡糖胺(GlcNAc)作为几丁质的水解机理,这种酶仅在外表面发生,而内部的结晶几丁质保持完好无损。几丁质中的N-乙酰基构成了强大的分子内和分子间氢键网络,这些网络有助于晶体结构的整合和屏障的形成,从而屏蔽了糖苷键与酸性催化剂的可及性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号