当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Perylenes by Oxidative Dimerization of Naphthalenes Bearing Radical Sources
ChemPlusChem ( IF 3.4 ) Pub Date : 2019-11-08 , DOI: 10.1002/cplu.201900620 Yasukazu Hirao 1 , Tomoki Okuda 1 , Yosuke Hamamoto 1 , Takashi Kubo 1
ChemPlusChem ( IF 3.4 ) Pub Date : 2019-11-08 , DOI: 10.1002/cplu.201900620 Yasukazu Hirao 1 , Tomoki Okuda 1 , Yosuke Hamamoto 1 , Takashi Kubo 1
Affiliation
|
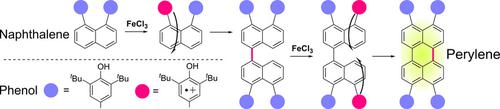
The introduction of phenol groups at the 1,8‐positions of naphthalene allowed its one‐pot oxidative dimerization and subsequent oxidative cyclization to the perylene structures under mild conditions and upon addition of iron (III) chloride. Examination using various derivatives and DFT calculations revealed that these reactions are initiated by the oxidation of the phenol moiety to generate a radical cation, which is supplied as a radical source for the reactions. The delocalization of the spin density from the radical cation of the introduced phenol groups to the naphthalene ring is proposed to be responsible for the intermolecular oxidative homo‐coupling reactions at the peri‐positions of the naphthalene and the subsequent oxidative cyclization at the 8,8’‐positions of the 1,1’‐binaphthyl derivative.
中文翻译:

通过带有自由基源的萘的氧化二聚反应形成Per
在萘的1,8位上引入酚基可以使其在温和条件下和加入氯化铁(III)时单锅氧化二聚,随后氧化环化成to结构。使用各种衍生物和DFT计算进行的检验表明,这些反应是由酚部分的氧化引发的,以生成自由基阳离子,该自由基阳离子作为反应的自由基源提供。有人认为,自旋密度从引入的酚基团的自由基阳离子到萘环的离域是造成萘周边分子间的氧化间均偶联反应以及随后在8,8处的氧化环化的原因。 1,-1'-联萘衍生物的'-位置。
更新日期:2019-11-08
中文翻译:

通过带有自由基源的萘的氧化二聚反应形成Per
在萘的1,8位上引入酚基可以使其在温和条件下和加入氯化铁(III)时单锅氧化二聚,随后氧化环化成to结构。使用各种衍生物和DFT计算进行的检验表明,这些反应是由酚部分的氧化引发的,以生成自由基阳离子,该自由基阳离子作为反应的自由基源提供。有人认为,自旋密度从引入的酚基团的自由基阳离子到萘环的离域是造成萘周边分子间的氧化间均偶联反应以及随后在8,8处的氧化环化的原因。 1,-1'-联萘衍生物的'-位置。


























 京公网安备 11010802027423号
京公网安备 11010802027423号