Computational and Theoretical Chemistry ( IF 2.8 ) Pub Date : 2019-10-19 , DOI: 10.1016/j.comptc.2019.112623 Yan-Xia Han , Li-Jie Hou , Qi Zhang , Bo-Wan Wu , Chao Kong , Zhi-Yuan Geng
|
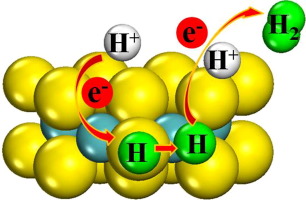
In this paper, the mechanisms of H2 evolution over the Mo-Edge of 2H-MoS2 with the unsaturated Mo (MoU) and S (SU) were investigated by the density functional theory. The calculations indicated that MoU was a key active site for H2 evolution. In the process of H2 evolution, the SU firstly absorbed H atom by the Volmer reaction, which caused the aggregation of electron on MoU, resulting in the barriers reduction of H transfer and H2 evolution on MoU. The formation of molybdenum hydride was the rate-limiting step. Compared to Mo-edge in 2H-MoS2, the barrier of key step for H2 evolution on Mo-edge with SU and MoU decreased obviously. This implied that the removal fractional S from the Mo-edge of 2H-MoS2 could enhance its H2 evolution activity and the electron trapping ability of active site for H2 evolution by the Volmer-Heyrovsky reaction might affect its activity.
中文翻译:

基于密度泛函理论的H 2生成对2H-MoS 2中Mo-Edge的不饱和Mo和S的生成机理
本文通过密度泛函理论研究了H 2在具有不饱和Mo(Mo U)和S(S U)的2H-MoS 2的Mo-Edge上演化的机理。计算表明,Mo U是H 2析出的关键活性位点。在H 2析出过程中,S U首先通过Volmer反应吸收H原子,从而引起电子在Mo U上聚集,从而减少了H迁移和Mo U上H 2析出的势垒。氢化钼的形成是限速步骤。与2H-MoS 2中的Mo-edge相比,S U和Mo U下Mo-edge上H 2析出关键步骤的障碍明显减少。这暗示着从2H-MoS 2的Mo边缘去除分数S可以增强其H 2的析出活性,而通过Volmer-Heyrovsky反应进行的H 2析出的活性位点的电子俘获能力可能会影响其活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号