当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional characterisation of two ferric-ion coordination modes of TtFbpA, the periplasmic subunit of an ABC-type iron transporter from Thermus thermophilus HB8.
Metallomics ( IF 3.4 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9mt00245f Peng Lu 1 , Yoshitaka Moriwaki , Mimin Zhang , Yukie Katayama , Yi Lu , Ken Okamoto , Tohru Terada , Kentaro Shimizu , Mengyao Wang , Takehiro Kamiya , Toru Fujiwara , Tomiko Asakura , Michio Suzuki , Etsuro Yoshimura , Koji Nagata
Metallomics ( IF 3.4 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9mt00245f Peng Lu 1 , Yoshitaka Moriwaki , Mimin Zhang , Yukie Katayama , Yi Lu , Ken Okamoto , Tohru Terada , Kentaro Shimizu , Mengyao Wang , Takehiro Kamiya , Toru Fujiwara , Tomiko Asakura , Michio Suzuki , Etsuro Yoshimura , Koji Nagata
Affiliation
|
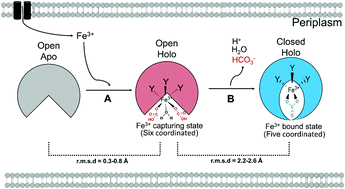
The ferric ion binding protein A of Thermus thermophilus HB8 (TtFbpA) is the periplasmic subunit of an ABC-type iron transporter. Two Fe3+-bound crystal structures at pH 5.5 and pH 7.5 and one apo structure have been reported for TtFbpA. In addition to three Tyr residues, TtFbpA coordinates with Fe3+ using two monodentate HCO3- and one H2O to form a six-coordinated mode at pH 5.5 or one bidentate CO32- to form a five-coordinated mode at pH 7.5. We investigated the biological significance of these Fe3+-bound forms of TtFbpA and the synergistic anions (HCO3- and CO32-). Quantum mechanical calculations in silico indicated that only these coordination modes were plausible out of six possibilities. Comparison of the crystal structures revealed a key motif, RZX1X2L(I/V), that would couple the Fe3+ coordination mode and the TtFbpA protein conformation. Both gel filtration chromatography and isothermal titration calorimetry showed that TtFbpA could bind Fe3+ at pH 7.5 but not at pH 5.5. Isothermal titration calorimetry also revealed that the binding at pH 7.5 was a three-step endothermic reaction that required NaHCO3. These results indicate that the holo structure at pH 5.5 is unstable in solution and may correspond to a transition state of TtFbpA-Fe3+ binding at pH 7.5 because HCO3- is much more abundant than CO32- at both pH values. Reorganisation of the synergistic ions and coupled protein conformational change will occur to form the stable TtFbpA-Fe3+ complex at pH 7.5, but not at pH 5.5. Identification of such a transition state will contribute to molecular design of novel FbpA inhibitors.
中文翻译:

TtFbpA的两个铁离子配位模式的功能表征,TtFbpA是嗜热栖热菌HB8的ABC型铁转运蛋白的周质亚基。
嗜热栖热菌HB8(TtFbpA)的铁离子结合蛋白A是ABC型铁转运蛋白的周质亚基。对于TtFbpA,已经报道了在pH 5.5和pH 7.5时有两个Fe3 +结合的晶体结构和一个载脂蛋白结构。除三个Tyr残基外,TtFbpA与Fe3 +配合使用两个单齿HCO3-和一个H2O在pH 5.5时形成六配位模式,或在pH 7.5时形成二齿CO32-形成五配位模式。我们研究了TtFbpA与这些Fe3 +结合形式和协同阴离子(HCO3-和CO32-)的生物学意义。计算机进行的量子力学计算表明,在六种可能性中,只有这些协调模式才是合理的。晶体结构的比较揭示了一个关键基序RZX1X2L(I / V),它将结合Fe3 +配位模式和TtFbpA蛋白构象。凝胶过滤色谱法和等温滴定量热法均显示,TtFbpA可以在pH 7.5时结合Fe3 +,而在pH 5.5时不结合。等温滴定量热法还显示,在pH 7.5下的结合是需要NaHCO3的三步吸热反应。这些结果表明,pH 5.5时的整体结构在溶液中不稳定,并且可能对应于pH 7.5时TtFbpA-Fe3 +结合的过渡态,因为在两个pH值下HCO3-比CO32-都丰富得多。在pH 7.5而不是pH 5.5时,将发生协同离子的重组和偶联的蛋白质构象变化,以形成稳定的TtFbpA-Fe3 +复合物。鉴定这种过渡态将有助于新型FbpA抑制剂的分子设计。
更新日期:2019-12-11
中文翻译:

TtFbpA的两个铁离子配位模式的功能表征,TtFbpA是嗜热栖热菌HB8的ABC型铁转运蛋白的周质亚基。
嗜热栖热菌HB8(TtFbpA)的铁离子结合蛋白A是ABC型铁转运蛋白的周质亚基。对于TtFbpA,已经报道了在pH 5.5和pH 7.5时有两个Fe3 +结合的晶体结构和一个载脂蛋白结构。除三个Tyr残基外,TtFbpA与Fe3 +配合使用两个单齿HCO3-和一个H2O在pH 5.5时形成六配位模式,或在pH 7.5时形成二齿CO32-形成五配位模式。我们研究了TtFbpA与这些Fe3 +结合形式和协同阴离子(HCO3-和CO32-)的生物学意义。计算机进行的量子力学计算表明,在六种可能性中,只有这些协调模式才是合理的。晶体结构的比较揭示了一个关键基序RZX1X2L(I / V),它将结合Fe3 +配位模式和TtFbpA蛋白构象。凝胶过滤色谱法和等温滴定量热法均显示,TtFbpA可以在pH 7.5时结合Fe3 +,而在pH 5.5时不结合。等温滴定量热法还显示,在pH 7.5下的结合是需要NaHCO3的三步吸热反应。这些结果表明,pH 5.5时的整体结构在溶液中不稳定,并且可能对应于pH 7.5时TtFbpA-Fe3 +结合的过渡态,因为在两个pH值下HCO3-比CO32-都丰富得多。在pH 7.5而不是pH 5.5时,将发生协同离子的重组和偶联的蛋白质构象变化,以形成稳定的TtFbpA-Fe3 +复合物。鉴定这种过渡态将有助于新型FbpA抑制剂的分子设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号