当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The influence of hydrofluoric acid etching processes on the photocatalytic hydrogen evolution reaction using mesoporous silicon nanoparticles.
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-10-16 , DOI: 10.1039/c9fd00098d Sarah A Martell 1 , Ulrike Werner-Zwanziger , Mita Dasog
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-10-16 , DOI: 10.1039/c9fd00098d Sarah A Martell 1 , Ulrike Werner-Zwanziger , Mita Dasog
Affiliation
|
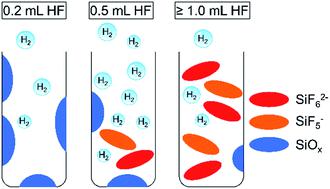
H2 has been identified as one of the potential energy vectors that can provide a sustainable energy supply when produced through solar-driven water-splitting reaction. Si is the second most abundant element in the Earth’s crust and can absorb a significant fraction of the solar spectrum while presenting little toxicity risk, making it an attractive material for photocatalytic H2 production. Hydrogen-terminated mesoporous Si (mp-Si) nanoparticles can be utilized to effectively drive the hydrogen evolution reaction using UV-to-visible light. In this work, the response of the photocatalytic activity of mp-Si nanoparticles to a series of HF acid treatments was investigated. A two-step magnesiothermic reduction method was used to prepare crystalline mp-Si nanoparticles with a specific surface area of 573 m2 g−1. The HF etching process was optimized as a function of the amount of acid added and the reaction time. The reaction time did not influence the H2 evolution rate substantially. However, the amount of HF used did have a significant effect on the photocatalytic activity. In the presence of ≥1.0 mL HF acid per 0.010 g of Si, morphological damage was observed using electron microscopy. N2 adsorption measurements indicated that the pore size and surface area were also altered. Solution-phase 19F{1H} NMR studies indicated the formation of SiF5− and SiF62− when larger volumes of HF were used. Both factors, morphological damage and the presence of byproducts in the pores, likely result in a lowering of the photocatalytic H2 evolution rate. Under the optimized HF treatment conditions (0.5 mL of HF per 0.010 g of Si), a H2 evolution rate of 1398 ± 30 μmol g−1 h−1 was observed.
中文翻译:

氢氟酸刻蚀工艺对使用介孔硅纳米粒子的光催化制氢反应的影响。
H 2被确定为当通过太阳能驱动的水分解反应产生时可以提供可持续能源供应的潜在能量载体之一。Si是地壳中含量第二高的元素,可以吸收大部分太阳光谱,同时几乎没有毒性风险,使其成为光催化H 2的有吸引力的材料生产。氢封端的介孔Si(mp-Si)纳米颗粒可用于利用紫外到可见光有效地驱动氢释放反应。在这项工作中,研究了mp-Si纳米颗粒的光催化活性对一系列HF酸处理的响应。采用两步法磁热还原法制备比表面积为573 m 2 g -1的晶状mp-Si纳米颗粒。根据添加的酸量和反应时间对HF蚀刻工艺进行了优化。反应时间不影响H 2进化率大大提高。但是,所使用的HF的量确实对光催化活性具有显着影响。在每0.010 g Si中存在≥1.0mL HF酸的情况下,使用电子显微镜观察到形态学破坏。N 2吸附测量表明,孔径和表面积也发生了变化。溶液相19 ˚F{ 1 H} NMR研究表明的SiF形成5 -和SIF 6 2-当使用更大的HF的量。形态破坏和孔中副产物的存在这两个因素都可能导致光催化H 2的降低进化率。在优化的HF处理条件下(每0.010 g硅0.5 mL HF),观察到H 2的释放速率为1398±30μmolg -1 h -1。
更新日期:2019-10-16
中文翻译:

氢氟酸刻蚀工艺对使用介孔硅纳米粒子的光催化制氢反应的影响。
H 2被确定为当通过太阳能驱动的水分解反应产生时可以提供可持续能源供应的潜在能量载体之一。Si是地壳中含量第二高的元素,可以吸收大部分太阳光谱,同时几乎没有毒性风险,使其成为光催化H 2的有吸引力的材料生产。氢封端的介孔Si(mp-Si)纳米颗粒可用于利用紫外到可见光有效地驱动氢释放反应。在这项工作中,研究了mp-Si纳米颗粒的光催化活性对一系列HF酸处理的响应。采用两步法磁热还原法制备比表面积为573 m 2 g -1的晶状mp-Si纳米颗粒。根据添加的酸量和反应时间对HF蚀刻工艺进行了优化。反应时间不影响H 2进化率大大提高。但是,所使用的HF的量确实对光催化活性具有显着影响。在每0.010 g Si中存在≥1.0mL HF酸的情况下,使用电子显微镜观察到形态学破坏。N 2吸附测量表明,孔径和表面积也发生了变化。溶液相19 ˚F{ 1 H} NMR研究表明的SiF形成5 -和SIF 6 2-当使用更大的HF的量。形态破坏和孔中副产物的存在这两个因素都可能导致光催化H 2的降低进化率。在优化的HF处理条件下(每0.010 g硅0.5 mL HF),观察到H 2的释放速率为1398±30μmolg -1 h -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号