当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
iPS Cells Attenuate Endothelial Leakage in Acute Lung Injury via TIMP-1 to Reduce FAK Activity
STEM CELLS ( IF 5.2 ) Pub Date : 2019-11-18 , DOI: 10.1002/stem.3093 Vincent Yi-Fong Su, Shih-Hwa Chiou, Chi-Shiuan Lin, Min-Hsiang Mo, Kuang-Yao Yang
STEM CELLS ( IF 5.2 ) Pub Date : 2019-11-18 , DOI: 10.1002/stem.3093 Vincent Yi-Fong Su, Shih-Hwa Chiou, Chi-Shiuan Lin, Min-Hsiang Mo, Kuang-Yao Yang
|
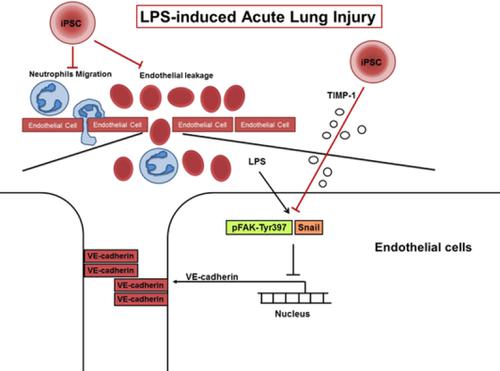
Induced pluripotent stem cells (iPSCs) can reduce the severity of endotoxin-induced acute lung injury (ALI). However, the interaction between iPSCs and vascular endothelium remains unclear. In this study, we investigated the effects of iPSCs in moderating pulmonary endothelial leakage in endotoxin-induced ALI. Murine iPSCs were delivered intravenously to male C57BL/6 mice (8-12 weeks old) 4 hours after intratracheal lipopolysaccharide (LPS) delivery. Histology, blood and bronchoalveolar lavage fluid (BALF) cytokine and junctional protein assays, and regulatory signaling pathway assays were performed 24 hours later. Human umbilical vein endothelial cells (HUVECs) were used as a model of junctional protein-expressing cells and stimulated with LPS. Our results showed that iPSC treatment alleviated histological signs of ALI, protein leakage, and proinflammatory cytokines. iPSC therapy restored vascular endothelial cadherin (VE-cadherin) expression in ALI mouse lungs. In HUVECs, human iPSCs (hiPSCs) restored disrupted VE-cadherin expression, and reduced the activity of Snail and focal adhesion kinase (FAK) phosphorylation in Tyr397 in response to LPS. iPSC conditioned medium contained extra anti-angiogenic factor of tissue inhibitor of metalloproteinases-1 (TIMP-1) compared to control medium. TIMP-1 inhibition diminished the beneficial effects of iPSC-CM in ALI mice. Our study suggested that iPSCs attenuate endothelial cell leakage in endotoxin-induced ALI via a mechanism involving TIMP-1 and the FAK/Snail pathway. © AlphaMed Press 2019 SIGNIFICANCE STATEMENT: Induced pluripotent stem cells (iPSCs) significantly diminished the severity of endotoxin-induced acute lung injury (ALI). However, the interaction between iPSCs and vascular endothelium remains unclear. This article found that iPSCs reduces the endothelial cell permeability by reversing LPS-induced suppression of VE-cadherin in pulmonary endothelium. This study further suggested that iPSCs down-regulate the expression of pFAK-Tyr397 and Snail via TIMP-1 in LPS-induced ALI.
中文翻译:

iPS 细胞通过 TIMP-1 减弱急性肺损伤中的内皮渗漏以降低 FAK 活性
诱导多能干细胞 (iPSC) 可以降低内毒素诱导的急性肺损伤 (ALI) 的严重程度。然而,iPSCs 和血管内皮之间的相互作用仍不清楚。在这项研究中,我们研究了 iPSCs 在内毒素诱导的 ALI 中调节肺内皮渗漏的作用。在气管内脂多糖 (LPS) 递送后 4 小时,将小鼠 iPSC 静脉注射给雄性 C57BL/6 小鼠(8-12 周龄)。24 小时后进行组织学、血液和支气管肺泡灌洗液 (BALF) 细胞因子和连接蛋白测定以及调节信号通路测定。人脐静脉内皮细胞 (HUVEC) 用作表达连接蛋白的细胞模型,并用 LPS 刺激。我们的结果表明,iPSC 治疗减轻了 ALI、蛋白质渗漏、和促炎细胞因子。iPSC 治疗恢复了 ALI 小鼠肺中血管内皮钙粘蛋白(VE-钙粘蛋白)的表达。在 HUVEC 中,人类 iPSC (hiPSC) 恢复了被破坏的 VE-钙粘蛋白表达,并降低了 Snail 的活性和 Tyr397 中粘着斑激酶 (FAK) 磷酸化以响应 LPS。与对照培养基相比,iPSC 条件培养基含有额外的金属蛋白酶组织抑制剂 1 (TIMP-1) 的抗血管生成因子。TIMP-1 抑制减弱了 iPSC-CM 在 ALI 小鼠中的有益作用。我们的研究表明,iPSCs 通过涉及 TIMP-1 和 FAK/Snail 通路的机制减弱内毒素诱导的 ALI 中的内皮细胞渗漏。© AlphaMed Press 2019 意义声明:诱导多能干细胞 (iPSC) 显着降低了内毒素诱导的急性肺损伤 (ALI) 的严重程度。然而,iPSCs 和血管内皮之间的相互作用仍不清楚。这篇文章发现 iPSCs 通过逆转 LPS 诱导的肺内皮中 VE-钙粘蛋白的抑制来降低内皮细胞通透性。该研究进一步表明,在 LPS 诱导的 ALI 中,iPSCs 通过 TIMP-1 下调 pFAK-Tyr397 和 Snail 的表达。
更新日期:2019-11-18
中文翻译:

iPS 细胞通过 TIMP-1 减弱急性肺损伤中的内皮渗漏以降低 FAK 活性
诱导多能干细胞 (iPSC) 可以降低内毒素诱导的急性肺损伤 (ALI) 的严重程度。然而,iPSCs 和血管内皮之间的相互作用仍不清楚。在这项研究中,我们研究了 iPSCs 在内毒素诱导的 ALI 中调节肺内皮渗漏的作用。在气管内脂多糖 (LPS) 递送后 4 小时,将小鼠 iPSC 静脉注射给雄性 C57BL/6 小鼠(8-12 周龄)。24 小时后进行组织学、血液和支气管肺泡灌洗液 (BALF) 细胞因子和连接蛋白测定以及调节信号通路测定。人脐静脉内皮细胞 (HUVEC) 用作表达连接蛋白的细胞模型,并用 LPS 刺激。我们的结果表明,iPSC 治疗减轻了 ALI、蛋白质渗漏、和促炎细胞因子。iPSC 治疗恢复了 ALI 小鼠肺中血管内皮钙粘蛋白(VE-钙粘蛋白)的表达。在 HUVEC 中,人类 iPSC (hiPSC) 恢复了被破坏的 VE-钙粘蛋白表达,并降低了 Snail 的活性和 Tyr397 中粘着斑激酶 (FAK) 磷酸化以响应 LPS。与对照培养基相比,iPSC 条件培养基含有额外的金属蛋白酶组织抑制剂 1 (TIMP-1) 的抗血管生成因子。TIMP-1 抑制减弱了 iPSC-CM 在 ALI 小鼠中的有益作用。我们的研究表明,iPSCs 通过涉及 TIMP-1 和 FAK/Snail 通路的机制减弱内毒素诱导的 ALI 中的内皮细胞渗漏。© AlphaMed Press 2019 意义声明:诱导多能干细胞 (iPSC) 显着降低了内毒素诱导的急性肺损伤 (ALI) 的严重程度。然而,iPSCs 和血管内皮之间的相互作用仍不清楚。这篇文章发现 iPSCs 通过逆转 LPS 诱导的肺内皮中 VE-钙粘蛋白的抑制来降低内皮细胞通透性。该研究进一步表明,在 LPS 诱导的 ALI 中,iPSCs 通过 TIMP-1 下调 pFAK-Tyr397 和 Snail 的表达。

























 京公网安备 11010802027423号
京公网安备 11010802027423号