Genetics in Medicine ( IF 8.8 ) Pub Date : 2019-10-08 , DOI: 10.1038/s41436-019-0667-y Ye Zhu 1, 2 , Kristi M Swanson 1 , Ricardo L Rojas 3 , Zhen Wang 1, 4 , Jennifer L St Sauver 1, 5 , Sue L Visscher 1 , Larry J Prokop 6 , Suzette J Bielinski 5 , Liewei Wang 7 , Richard Weinshilboum 7 , Bijan J Borah 1, 2
|
Purpose
To examine the evidence on the cost-effectiveness of implementing pharmacogenomics (PGx) in cardiovascular disease (CVD) care.
Methods
We conducted a systematic review using multiple databases from inception to 2018. The titles and abstracts of cost-effectiveness studies on PGx-guided treatment in CVD care were screened, and full texts were extracted.
Results
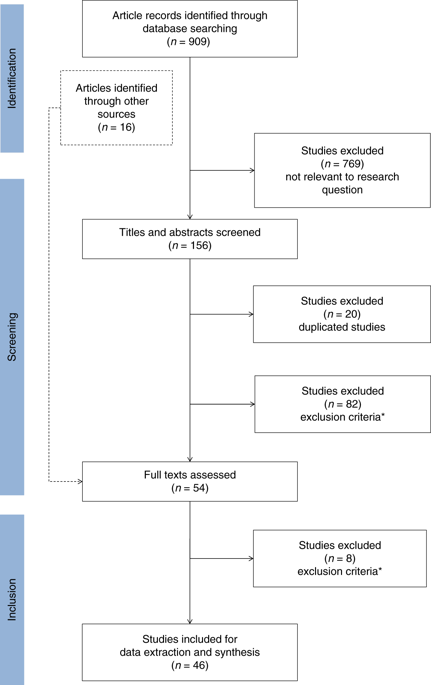
We screened 909 studies and included 46 to synthesize. Acute coronary syndrome and atrial fibrillation were the predominantly studied conditions (59%). Most studies (78%) examined warfarin–CYP2C9/VKORC1 or clopidogrel–CYP2C19. A payer’s perspective was commonly used (39%) for cost calculations, and most studies (46%) were US-based. The majority (67%) of the studies found PGx testing to be cost-effective in CVD care, but cost-effectiveness varied across drugs and conditions. Two studies examined PGx panel testing, of which one examined pre-emptive testing strategies.
Conclusion
We found mixed evidence on the cost-effectiveness of PGx in CVD care. Supportive evidence exists for clopidogrel–CYP2C19 and warfarin–CYP2C9/VKORC1, but evidence is limited in other drug–gene combinations. Gaps persist, including unclear explanation of perspective and cost inputs, underreporting of study design elements critical to economic evaluations, and limited examination of PGx panel and pre-emptive testing for their cost-effectiveness. This review identifies the need for further research on economic evaluations of PGx implementation.
中文翻译:

对药物基因组学指导的心血管疾病治疗成本效益证据的系统评价。
目的
研究在心血管疾病 (CVD) 护理中实施药物基因组学 (PGx) 的成本效益的证据。
方法
我们使用多个数据库从开始到 2018 年进行了系统评价。筛选了 PGx 指导的 CVD 护理治疗成本效益研究的标题和摘要,并提取了全文。
结果
我们筛选了 909 项研究,并纳入了 46 项进行综合。急性冠状动脉综合征和心房颤动是主要研究的疾病(59%)。大多数研究 (78%) 检测了华法林-CYP2C9/VKORC1或氯吡格雷-CYP2C19。付款人的观点通常用于成本计算(39%),大多数研究(46%)是基于美国的。大多数 (67%) 研究发现 PGx 检测在 CVD 护理中具有成本效益,但成本效益因药物和条件而异。两项研究检查了 PGx 小组测试,其中一项研究了先发制人的测试策略。
结论
我们发现关于 PGx 在 CVD 护理中的成本效益的混合证据。氯吡格雷-CYP2C19和华法林-CYP2C9/VKORC1存在支持性证据,但其他药物-基因组合的证据有限。差距仍然存在,包括对视角和成本投入的解释不明确,对经济评估至关重要的研究设计要素报告不足,以及对 PGx 小组的有限审查和先发制人的成本效益测试。本综述确定了对 PGx 实施的经济评估进行进一步研究的必要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号