Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients With Septic Shock
JAMA ( IF 120.7 ) Pub Date : 2019-10-15 , DOI: 10.1001/jama.2019.14607 Pierre-Francois Laterre 1 , Scott M Berry 2 , Allan Blemings 3 , Jan E Carlsen 4 , Bruno François 5 , Todd Graves 2 , Karsten Jacobsen 3 , Roger J Lewis 2, 6, 7, 8 , Steven M Opal 9 , Anders Perner 10 , Peter Pickkers 11 , James A Russell 12 , Nis A Windeløv 3 , Donald M Yealy 13 , Pierre Asfar 14 , Morten H Bestle 15 , Grégoire Muller 16 , Cédric Bruel 17 , Noëlle Brulé 18 , Johan Decruyenaere 19 , Alain-Michel Dive 20 , Thierry Dugernier 21 , Kenneth Krell 22 , Jean-Yves Lefrant 23 , Bruno Megarbane 24 , Emmanuelle Mercier 25 , Jean-Paul Mira 26 , Jean-Pierre Quenot 27 , Bodil Steen Rasmussen 28 , Hans-Christian Thorsen-Meyer 10 , Margot Vander Laenen 29 , Marianne Lauridsen Vang 30 , Philippe Vignon 5 , Isabelle Vinatier 31 , Sine Wichmann 32 , Xavier Wittebole 1 , Anne Louise Kjølbye 3 , Derek C Angus 33, 34 ,
JAMA ( IF 120.7 ) Pub Date : 2019-10-15 , DOI: 10.1001/jama.2019.14607 Pierre-Francois Laterre 1 , Scott M Berry 2 , Allan Blemings 3 , Jan E Carlsen 4 , Bruno François 5 , Todd Graves 2 , Karsten Jacobsen 3 , Roger J Lewis 2, 6, 7, 8 , Steven M Opal 9 , Anders Perner 10 , Peter Pickkers 11 , James A Russell 12 , Nis A Windeløv 3 , Donald M Yealy 13 , Pierre Asfar 14 , Morten H Bestle 15 , Grégoire Muller 16 , Cédric Bruel 17 , Noëlle Brulé 18 , Johan Decruyenaere 19 , Alain-Michel Dive 20 , Thierry Dugernier 21 , Kenneth Krell 22 , Jean-Yves Lefrant 23 , Bruno Megarbane 24 , Emmanuelle Mercier 25 , Jean-Paul Mira 26 , Jean-Pierre Quenot 27 , Bodil Steen Rasmussen 28 , Hans-Christian Thorsen-Meyer 10 , Margot Vander Laenen 29 , Marianne Lauridsen Vang 30 , Philippe Vignon 5 , Isabelle Vinatier 31 , Sine Wichmann 32 , Xavier Wittebole 1 , Anne Louise Kjølbye 3 , Derek C Angus 33, 34 ,
Affiliation
|
Importance
Norepinephrine, the first-line vasopressor for septic shock, is not always effective and has important catecholaminergic adverse effects. Selepressin, a selective vasopressin V1a receptor agonist, is a noncatecholaminergic vasopressor that may mitigate sepsis-induced vasodilatation, vascular leakage, and edema, with fewer adverse effects. Objective
To test whether selepressin improves outcome in septic shock. Design, Setting, and Participants
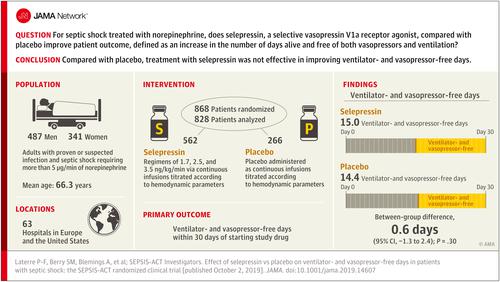
An adaptive phase 2b/3 randomized clinical trial comprising 2 parts that included adult patients (n = 868) with septic shock requiring more than 5 μg/min of norepinephrine. Part 1 used a Bayesian algorithm to adjust randomization probabilities to alternative selepressin dosing regimens and to trigger transition to part 2, which would compare the best-performing regimen with placebo. The trial was conducted between July 2015 and August 2017 in 63 hospitals in Belgium, Denmark, France, the Netherlands, and the United States, and follow-up was completed by May 2018. Interventions
Random assignment to 1 of 3 dosing regimens of selepressin (starting infusion rates of 1.7, 2.5, and 3.5 ng/kg/min; n = 585) or to placebo (n = 283), all administered as continuous infusions titrated according to hemodynamic parameters. Main Outcomes and Measures
Primary end point was ventilator- and vasopressor-free days within 30 days (deaths assigned zero days) of commencing study drug. Key secondary end points were 90-day mortality, kidney replacement therapy-free days, and ICU-free days. Results
Among 868 randomized patients, 828 received study drug (mean age, 66.3 years; 341 [41.2%] women) and comprised the primary analysis cohort, of whom 562 received 1 of 3 selepressin regimens, 266 received placebo, and 817 (98.7%) completed the trial. The trial was stopped for futility at the end of part 1. Median study drug duration was 37.8 hours (IQR, 17.8-72.4). There were no significant differences in the primary end point (ventilator- and vasopressor-free days: 15.0 vs 14.5 in the selepressin and placebo groups; difference, 0.6 [95% CI, -1.3 to 2.4]; P = .30) or key secondary end points (90-day mortality, 40.6% vs 39.4%; difference, 1.1% [95% CI, -6.5% to 8.8%]; P = .77; kidney replacement therapy-free days: 18.5 vs 18.2; difference, 0.3 [95% CI, -2.1 to 2.6]; P = .85; ICU-free days: 12.6 vs 12.2; difference, 0.5 [95% CI, -1.2 to 2.2]; P = .41). Adverse event rates included cardiac arrhythmias (27.9% vs 25.2% of patients), cardiac ischemia (6.6% vs 5.6%), mesenteric ischemia (3.2% vs 2.6%), and peripheral ischemia (2.3% vs 2.3%). Conclusions and Relevance
Among patients with septic shock receiving norepinephrine, administration of selepressin, compared with placebo, did not result in improvement in vasopressor- and ventilator-free days within 30 days. Further research would be needed to evaluate the potential role of selepressin for other patient-centered outcomes in septic shock. Trial Registration
ClinicalTrials.gov Identifier: NCT02508649.
中文翻译:

Selepressin 与安慰剂对感染性休克患者无呼吸机和无血管加压药天数的影响
重要性 去甲肾上腺素是脓毒性休克的一线升压药,但并不总是有效,并且具有重要的儿茶酚胺能副作用。Selepressin 是一种选择性加压素 V1a 受体激动剂,是一种非儿茶酚胺能血管加压剂,可减轻脓毒症引起的血管舒张、血管渗漏和水肿,副作用较少。目的检测selepressin是否能改善感染性休克的预后。设计、设置和参与者 一项适应性 2b/3 期随机临床试验包括 2 个部分,其中包括成人患者 (n = 868),感染性休克需要超过 5 μg/min 的去甲肾上腺素。第 1 部分使用贝叶斯算法来调整随机化概率以替代 selepressin 给药方案,并触发过渡到第 2 部分,该部分将比较表现最佳的方案与安慰剂。该试验于 2015 年 7 月至 2017 年 8 月在比利时、丹麦、法国、荷兰和美国的 63 家医院进行,随访于 2018 年 5 月完成。起始输注速率为 1.7、2.5 和 3.5 ng/kg/min;n = 585) 或安慰剂 (n = 283),均根据血流动力学参数滴定连续输注。主要结果和措施 主要终点是开始研究药物后 30 天内(死亡分配为零天)内无呼吸机和血管加压药的天数。关键的次要终点是 90 天死亡率、无肾脏替代治疗天数和无 ICU 天数。结果 在 868 名随机患者中,828 名接受了研究药物治疗(平均年龄 66.3 岁;341 名 [41.2%] 女性)并构成主要分析队列,其中 562 人接受了 3 种 Selepressin 方案中的 1 种,266 人接受安慰剂,817 人(98.7%)完成了试验。该试验在第 1 部分结束时因无效而停止。中位研究药物持续时间为 37.8 小时(IQR,17.8-72.4)。主要终点(无呼吸机和无血管加压药的天数:15.0 vs 14.5,selepressin 组和安慰剂组;差异,0.6 [95% CI,-1.3 至 2.4];P = .30)或关键终点没有显着差异次要终点(90 天死亡率,40.6% vs 39.4%;差异,1.1% [95% CI,-6.5% 至 8.8%];P = .77;无肾脏替代治疗天数:18.5 vs 18.2;差异, 0.3 [95% CI,-2.1 至 2.6];P = .85;无 ICU 天数:12.6 对 12.2;差异,0.5 [95% CI,-1.2 至 2.2];P = .41)。不良事件发生率包括心律失常(27.9% 对 25.2% 的患者)、心脏缺血(6.6% 对 5.6%)、肠系膜缺血(3. 2% 对 2.6%)和外周缺血(2.3% 对 2.3%)。结论和相关性 在接受去甲肾上腺素治疗的感染性休克患者中,与安慰剂相比,使用 selepressin 并没有改善 30 天内无血管加压药和无呼吸机的天数。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。
更新日期:2019-10-15
中文翻译:

Selepressin 与安慰剂对感染性休克患者无呼吸机和无血管加压药天数的影响
重要性 去甲肾上腺素是脓毒性休克的一线升压药,但并不总是有效,并且具有重要的儿茶酚胺能副作用。Selepressin 是一种选择性加压素 V1a 受体激动剂,是一种非儿茶酚胺能血管加压剂,可减轻脓毒症引起的血管舒张、血管渗漏和水肿,副作用较少。目的检测selepressin是否能改善感染性休克的预后。设计、设置和参与者 一项适应性 2b/3 期随机临床试验包括 2 个部分,其中包括成人患者 (n = 868),感染性休克需要超过 5 μg/min 的去甲肾上腺素。第 1 部分使用贝叶斯算法来调整随机化概率以替代 selepressin 给药方案,并触发过渡到第 2 部分,该部分将比较表现最佳的方案与安慰剂。该试验于 2015 年 7 月至 2017 年 8 月在比利时、丹麦、法国、荷兰和美国的 63 家医院进行,随访于 2018 年 5 月完成。起始输注速率为 1.7、2.5 和 3.5 ng/kg/min;n = 585) 或安慰剂 (n = 283),均根据血流动力学参数滴定连续输注。主要结果和措施 主要终点是开始研究药物后 30 天内(死亡分配为零天)内无呼吸机和血管加压药的天数。关键的次要终点是 90 天死亡率、无肾脏替代治疗天数和无 ICU 天数。结果 在 868 名随机患者中,828 名接受了研究药物治疗(平均年龄 66.3 岁;341 名 [41.2%] 女性)并构成主要分析队列,其中 562 人接受了 3 种 Selepressin 方案中的 1 种,266 人接受安慰剂,817 人(98.7%)完成了试验。该试验在第 1 部分结束时因无效而停止。中位研究药物持续时间为 37.8 小时(IQR,17.8-72.4)。主要终点(无呼吸机和无血管加压药的天数:15.0 vs 14.5,selepressin 组和安慰剂组;差异,0.6 [95% CI,-1.3 至 2.4];P = .30)或关键终点没有显着差异次要终点(90 天死亡率,40.6% vs 39.4%;差异,1.1% [95% CI,-6.5% 至 8.8%];P = .77;无肾脏替代治疗天数:18.5 vs 18.2;差异, 0.3 [95% CI,-2.1 至 2.6];P = .85;无 ICU 天数:12.6 对 12.2;差异,0.5 [95% CI,-1.2 至 2.2];P = .41)。不良事件发生率包括心律失常(27.9% 对 25.2% 的患者)、心脏缺血(6.6% 对 5.6%)、肠系膜缺血(3. 2% 对 2.6%)和外周缺血(2.3% 对 2.3%)。结论和相关性 在接受去甲肾上腺素治疗的感染性休克患者中,与安慰剂相比,使用 selepressin 并没有改善 30 天内无血管加压药和无呼吸机的天数。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。需要进一步的研究来评估 selepressin 在感染性休克中其他以患者为中心的结果的潜在作用。试验注册 ClinicalTrials.gov 标识符:NCT02508649。


























 京公网安备 11010802027423号
京公网安备 11010802027423号