Computational and Theoretical Chemistry ( IF 2.8 ) Pub Date : 2019-09-30 , DOI: 10.1016/j.comptc.2019.112605 Rui Zhao , Li Sheng , Kunqi Gao
|
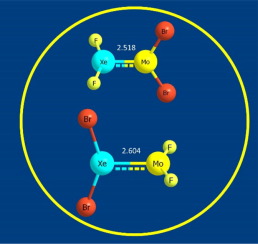
The X2XeMoY2 (X = F, Cl, Br; Y = F, Cl, Br) compounds were investigated at the B3LYP/Aug-cc-pVTZ level of theory. The calculated molecular structures suggested that the Xe and Mo atoms form a double bond, which supported by the molecular orbital (MO) analysis. Our results showed that in the X2XeMoY2 molecules, the Xe-Mo bond length is shorter when the electronegativity of X atom is stronger and the electronegativity of Y atom is weaker. Interestingly, an unexpectedly short Mo-Y bond was found in the X2XeMoY2 compound, and the MO analysis indicates the three atoms (Y-Mo-Y) form a 3c/4e bond.
中文翻译:

卤素原子如何影响Xe-Mo双键?X 2 XeMoY 2(X = F,Cl,Br; Y = F,Cl,Br)的理论研究
在理论上的B3LYP / Aug-cc-pVTZ水平下研究了X 2 XeMoY 2(X = F,Cl,Br; Y = F,Cl,Br)化合物。计算得出的分子结构表明,Xe和Mo原子形成一个双键,并由分子轨道(MO)分析支持。我们的结果表明,在X 2 XeMoY 2分子中,X原子的电负性越强,而Y原子的电负性越弱,Xe-Mo键长越短。有趣的是,在X 2 XeMoY 2化合物中发现了一个意想不到的短Mo-Y键,MO分析表明这三个原子(Y-Mo-Y)形成3c / 4e键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号