当前位置:
X-MOL 学术
›
J. Polym. Sci. A Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Helicity Induction and Its Static Memory of Poly(biphenylylacetylene)s Bearing Pyridine N-Oxide Groups and Their Use as Asymmetric Organocatalysts
Journal of Polymer Science Part A: Polymer Chemistry ( IF 2.869 ) Pub Date : 2019-09-30 , DOI: 10.1002/pola.29501 Mitsuka Ando 1 , Ryoma Ishidate 2 , Tomoyuki Ikai 1 , Katsuhiro Maeda 3, 4 , Eiji Yashima 1, 2
Journal of Polymer Science Part A: Polymer Chemistry ( IF 2.869 ) Pub Date : 2019-09-30 , DOI: 10.1002/pola.29501 Mitsuka Ando 1 , Ryoma Ishidate 2 , Tomoyuki Ikai 1 , Katsuhiro Maeda 3, 4 , Eiji Yashima 1, 2
Affiliation
|
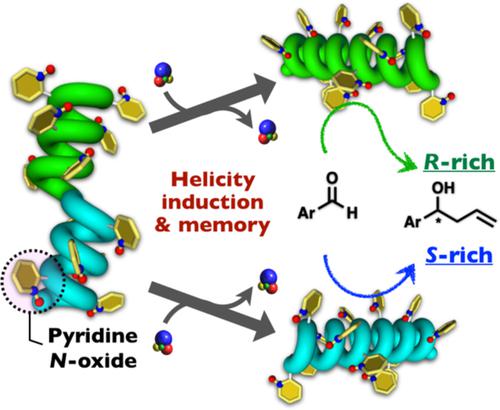
Novel poly(biphenylylacetylene) derivatives carrying different types of pyridine N-oxide units with a bulky or less-bulky substituent at a different position as the functional pendant groups (poly-2a and poly-2b) were synthesized by the rhodium-catalyzed polymerization of the corresponding monomers. The influence of the steric environment around the catalytically active pyridine N-oxide sites on the helicity induction and its static memory as well as the asymmetric catalytic activities of the resulting helical polymers with a macromolecular helicity memory was investigated. The polyacetylenes formed an excess one-handed helical conformation upon noncovalent interactions with optically active alcohols and the induced macromolecular helicities of the polyacetylenes were efficiently memorized after the removal of the chiral inducers. Poly-2b with the macromolecular helicity memory showed an enantioselectivity for the catalytic asymmetric allylation of benzaldehydes, producing optically active allyl alcohols, although their enantioselectivities were low. On the other hand, poly-2a exhibited a negligible catalytic activity probably due to the bulky substituent at the o-position of the pyridine N-oxide residues, while poly-2a underwent a unique helix-inversion with the increasing concentration of chiral alcohols and the opposite helicity of poly-2a was further successfully memorized. © 2019 Wiley Periodicals, Inc. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 2481–2490
中文翻译:

带有吡啶N-氧化物基团的聚(联苯乙炔)的螺旋感应及其静态记忆及其作为不对称有机催化剂的应用
通过铑催化聚合合成了新型聚(联苯乙炔)衍生物,其带有不同类型的吡啶N-氧化物单元,在不同的位置作为功能侧基(poly- 2a和 poly - 2b )具有体积较大或体积较小的取代基。相应的单体。催化活性吡啶N周围空间环境的影响研究了螺旋性诱导及其静态记忆上的-氧化物位点以及所得具有大分子螺旋性记忆的螺旋聚合物的不对称催化活性。聚乙炔在与光学活性醇的非共价相互作用时形成过量的单向螺旋构象,并且在去除手性诱导剂后有效地记忆聚乙炔的诱导大分子螺旋。具有大分子螺旋性记忆的聚2b对苯甲醛的催化不对称烯丙基化显示出对映选择性,产生光学活性烯丙醇,尽管它们的对映选择性很低。另一方面,poly- 2a表现出可忽略不计的催化活性可能是由于吡啶N-氧化物残基的o位上的大量取代基,而随着手性醇浓度的增加,聚2a经历了独特的螺旋反转,而聚2a的相反螺旋度是进一步成功记忆。© 2019 Wiley Periodicals, Inc. J. Polym。科学,A 部分:Polym。化学。2019 , 57 , 2481–2490
更新日期:2019-09-30
中文翻译:

带有吡啶N-氧化物基团的聚(联苯乙炔)的螺旋感应及其静态记忆及其作为不对称有机催化剂的应用
通过铑催化聚合合成了新型聚(联苯乙炔)衍生物,其带有不同类型的吡啶N-氧化物单元,在不同的位置作为功能侧基(poly- 2a和 poly - 2b )具有体积较大或体积较小的取代基。相应的单体。催化活性吡啶N周围空间环境的影响研究了螺旋性诱导及其静态记忆上的-氧化物位点以及所得具有大分子螺旋性记忆的螺旋聚合物的不对称催化活性。聚乙炔在与光学活性醇的非共价相互作用时形成过量的单向螺旋构象,并且在去除手性诱导剂后有效地记忆聚乙炔的诱导大分子螺旋。具有大分子螺旋性记忆的聚2b对苯甲醛的催化不对称烯丙基化显示出对映选择性,产生光学活性烯丙醇,尽管它们的对映选择性很低。另一方面,poly- 2a表现出可忽略不计的催化活性可能是由于吡啶N-氧化物残基的o位上的大量取代基,而随着手性醇浓度的增加,聚2a经历了独特的螺旋反转,而聚2a的相反螺旋度是进一步成功记忆。© 2019 Wiley Periodicals, Inc. J. Polym。科学,A 部分:Polym。化学。2019 , 57 , 2481–2490


























 京公网安备 11010802027423号
京公网安备 11010802027423号